9.E: Ejercicios
- ID de página
- 24789
Estos son ejercicios de tarea para acompañar el Mapa de texto creado para “Química: La ciencia central” por Brown et al. Se pueden encontrar bancos de preguntas de química general complementaria para otros mapas de texto y se puede acceder aquí . Además de estas preguntas disponibles públicamente, el acceso al banco privado de problemas para su uso en exámenes y tareas está disponible para los profesores solo de manera individual; comuníquese con Delmar Larsen para obtener una cuenta con permiso de acceso.
9.1: FORMAS MOLECULARES
9.2: EL MODELO VSEPR
Problemas conceptuales
-
¿Cuál es la principal diferencia entre el modelo VSEPR y las estructuras electrónicas de Lewis?
-
¿Cuáles son las diferencias entre la geometría molecular y las estructuras electrónicas de Lewis? ¿Pueden dos moléculas con las mismas estructuras electrónicas de Lewis tener geometrías moleculares diferentes? ¿Pueden dos moléculas con la misma geometría molecular tener diferentes estructuras de electrones de Lewis? En cada caso, respalde su respuesta con un ejemplo.
-
¿Cómo aborda el modelo VSEPR la presencia de enlaces múltiples?
-
Tres moléculas tienen las siguientes fórmulas genéricas: AX 2 , AX 2 E y AX 2 E 2 . Predecir la geometría molecular de cada uno y organizarlos en orden creciente de ángulo X – A – X.
-
¿Cuál tiene los ángulos más pequeños alrededor del átomo central: H 2 S o SiH 4 ? ¿Por qué? ¿Las estructuras electrónicas de Lewis de estas moléculas predicen cuál tiene el ángulo más pequeño?
-
Discute en tus propias palabras por qué los pares de electrones solitarios ocupan más espacio que los pares de enlace. ¿Cómo afecta la presencia de pares solitarios a la geometría molecular?
-
Cuando se usa VSEPR para predecir la geometría molecular, la importancia de las repulsiones entre pares de electrones disminuye en el siguiente orden: LP – LP, LP – BP, BP – BP. Explica este orden. Dibuje estructuras de moléculas reales que muestren por separado cada una de estas interacciones.
-
¿Cómo afectan los enlaces múltiples a la geometría molecular? ¿Un enlace múltiple ocupa más o menos espacio alrededor de un átomo que un enlace simple? un par solitario?
-
Los alcanos de cadena recta no tienen estructuras lineales pero están “doblados”. Usando n -hexano como ejemplo, explique por qué esto es así. Compare la geometría del 1-hexeno con la de n -hexano.
-
¿Cómo se relaciona la geometría molecular con la presencia o ausencia de un momento dipolar molecular?
-
¿Cómo se relacionan la geometría molecular y los momentos dipolares con propiedades físicas como el punto de fusión y el punto de ebullición?
-
¿Qué dos características de la estructura y la unión de una molécula se requieren para que una molécula se considere polar? ¿Es probable que COF 2 tenga un momento dipolar significativo? Explica tu respuesta.
-
Cuando un químico dice que una molécula es polar , ¿qué significa esto? ¿Cuáles son las propiedades físicas generales de las moléculas polares?
-
Usa el modelo VSPER y tu conocimiento de los momentos de unión y dipolo para predecir qué moléculas serán líquidas o sólidas a temperatura ambiente y cuáles serán gases. Explique su justificación para cada opción. Justifica tus respuestas.
- CH 3 Cl
- PCl 3
- CO
- SF 6
- SI 5
- CH 3 OCH 3
- CCl 3 H
- H 3 COH
-
The idealized molecular geometry of BrF 5 is square pyramidal, with one lone pair. What effect does the lone pair have on the actual molecular geometry of BrF 5 ? If LP–BP repulsions were weaker than BP–BP repulsions, what would be the effect on the molecular geometry of BrF 5 ?
-
Which has the smallest bond angle around the central atom—H 2 S, H 2 Se, or H 2 Te? the largest? Justify your answers.
-
Which of these molecular geometries always results in a molecule with a net dipole moment: linear, bent, trigonal planar, tetrahedral, seesaw, trigonal pyramidal, square pyramidal, and octahedral? For the geometries that do not always produce a net dipole moment, what factor(s) will result in a net dipole moment?
Conceptual Answers
-
-
-
To a first approximation, the VSEPR model assumes that multiple bonds and single bonds have the same effect on electron pair geometry and molecular geometry; in other words, VSEPR treats multiple bonds like single bonds. Only when considering fine points of molecular structure does VSEPR recognize that multiple bonds occupy more space around the central atom than single bonds.
-
-
-
-
-
-
-
-
Physical properties like boiling point and melting point depend upon the existence and magnitude of the dipole moment of a molecule. In general, molecules that have substantial dipole moments are likely to exhibit greater intermolecular interactions, resulting in higher melting points and boiling points.
-
-
The term “polar” is generally used to mean that a molecule has an asymmetrical structure and contains polar bonds. The resulting dipole moment causes the substance to have a higher boiling or melting point than a nonpolar substance.
Numerical Problems
-
Give the number of electron groups around the central atom and the molecular geometry for each molecule. Classify the electron groups in each species as bonding pairs or lone pairs.
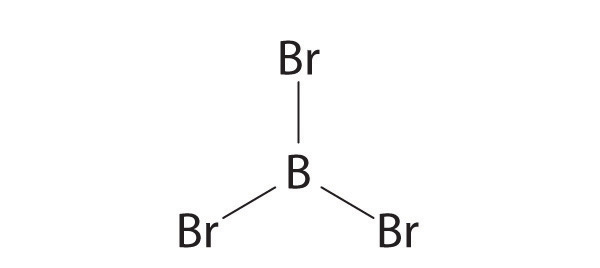
- BF 3
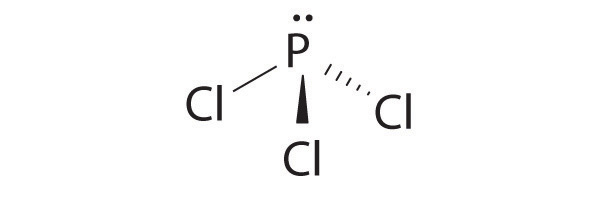
- PCl 3
- XeF 2
- AlCl 4 −
- CH 2 Cl 2
-
Give the number of electron groups around the central atom and the molecular geometry for each species. Classify the electron groups in each species as bonding pairs or lone pairs.
- ICl 3
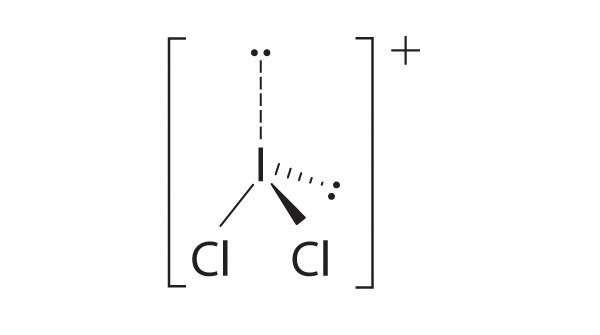
- CCl 3 +
- H 2 Te
- XeF 4
- NH 4 +
-
Give the number of electron groups around the central atom and the molecular geometry for each molecule. For structures that are not linear, draw three-dimensional representations, clearly showing the positions of the lone pairs of electrons.
- HCl
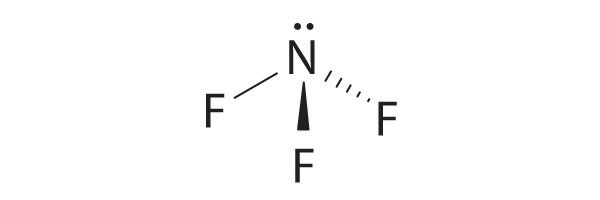
- NF 3
- ICl 2 +
- N 3 −
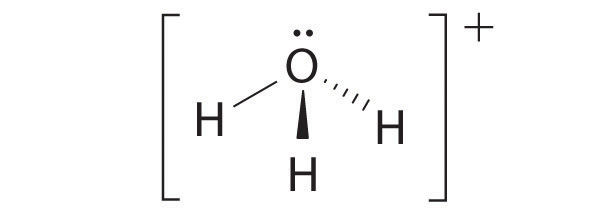
- H 3 O +
-
Give the number of electron groups around the central atom and the molecular geometry for each molecule. For structures that are not linear, draw three-dimensional representations, clearly showing the positions of the lone pairs of electrons.
- SO 3
- NH 2 −
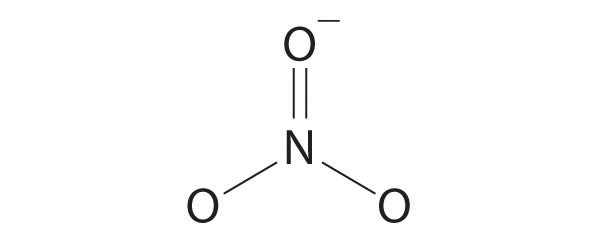
- NO 3 −
- I 3 −
- OF 2
-
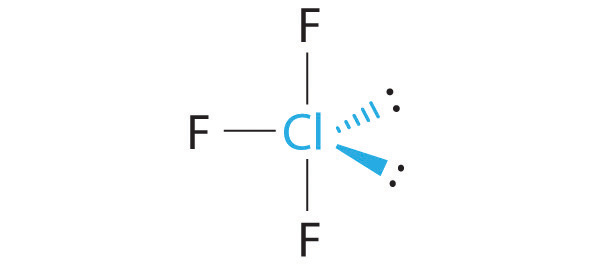
What is the molecular geometry of ClF 3 ? Draw a three-dimensional representation of its structure and explain the effect of any lone pairs on the idealized geometry.
-
Predict the molecular geometry of each of the following.
- ICl 3
- AsF 5
- NO 2 −
- TeCl 4
-
Predict whether each molecule has a net dipole moment. Justify your answers and indicate the direction of any bond dipoles.
- NO
- HF
- PCl 3
- CO 2
- SO 2
- SF 4
-
Predict whether each molecule has a net dipole moment. Justify your answers and indicate the direction of any bond dipoles.
- OF 2
- BCl 3
- CH 2 Cl 2
- TeF 4
- CH 3 OH
- XeO 4
-
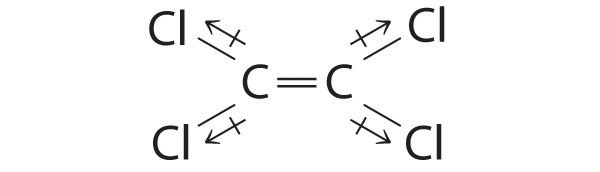
Of the molecules Cl 2 C=Cl 2 , IF 3 , and SF 6 , which has a net dipole moment? Explain your reasoning.
-
Of the molecules SO 3 , XeF 4 , and H 2 C=Cl 2 , which has a net dipole moment? Explain your reasoning.
Numerical Answers
-
- trigonal planar (all electron groups are bonding pairs)
- tetrahedral (one lone pair on P)
- trigonal bipyramidal (three lone pairs on Xe)
- tetrahedral (all electron groups on Al are bonding pairs)
- tetrahedral (all electron groups on C are bonding pairs)
-
-
- four electron groups, linear molecular geometry
-
four electron groups, pyramidal molecular geometry
-
four electron groups, bent molecular geometry
- two electron groups, linear molecular geometry
-
four electron groups, pyramidal molecular geometry
-
-
The idealized geometry is T shaped, but the two lone pairs of electrons on Cl will distort the structure, making the F–Cl–F angle less than 180°.
-
-
-
-
Cl 2 C=CCl 2 : Although the C–Cl bonds are rather polar, the individual bond dipoles cancel one another in this symmetrical structure, and Cl 2 C=CCl 2 does not have a net dipole moment.
IF 3 : In this structure, the individual I–F bond dipoles cannot cancel one another, giving IF 3 a net dipole moment.
SF 6 : The S–F bonds are quite polar, but the individual bond dipoles cancel one another in an octahedral structure. Thus, SF 6 has no net dipole moment.
9.3: MOLECULAR SHAPE AND MOLECULAR POLARITY
Conceptual Problems
- Why do ionic compounds such as KI exhibit substantially less than 100% ionic character in the gas phase?
- Of the compounds LiI and LiF, which would you expect to behave more like a classical ionic compound? Which would have the greater dipole moment in the gas phase? Explain your answers.
Numerical Problems
-
Predict whether each compound is purely covalent, purely ionic, or polar covalent.
- RbCl
- S 8
- TiCl 2
- SbCl 3
- LiI
- Br 2
-
Based on relative electronegativities, classify the bonding in each compound as ionic, covalent, or polar covalent. Indicate the direction of the bond dipole for each polar covalent bond.
- NO
- HF
- MgO
- AlCl 3
- SiO 2
- the C=O bond in acetone
- O 3
-
Based on relative electronegativities, classify the bonding in each compound as ionic, covalent, or polar covalent. Indicate the direction of the bond dipole for each polar covalent bond.
- NaBr
- OF 2
- BCl 3
- the S–S bond in CH 3 CH 2 SSCH 2 CH 3
- the C–Cl bond in CH 2 Cl 2
- the O–H bond in CH 3 OH
- PtCl 4 2−
-
Classify each species as having 0%–40% ionic character, 40%–60% ionic character, or 60%–100% ionic character based on the type of bonding you would expect. Justify your reasoning.
- CaO
- S 8
- AlBr 3
- ICl
- Na 2 S
- SiO 2
- LiBr
-
If the bond distance in HCl (dipole moment = 1.109 D) were double the actual value of 127.46 pm, what would be the effect on the charge localized on each atom? What would be the percent negative charge on Cl? At the actual bond distance, how would doubling the charge on each atom affect the dipole moment? Would this represent more ionic or covalent character?
-
Calculate the percent ionic character of HF (dipole moment = 1.826 D) if the H–F bond distance is 92 pm.
-
Calculate the percent ionic character of CO (dipole moment = 0.110 D) if the C–O distance is 113 pm.
-
Calculate the percent ionic character of PbS and PbO in the gas phase, given the following information: for PbS, r = 228.69 pm and µ = 3.59 D; for PbO, r = 192.18 pm and µ = 4.64 D. Would you classify these compounds as having covalent or polar covalent bonds in the solid state?
9.4: COVALENT BONDING AND ORBITAL OVERLAP
9.5: HYBRID ORBITALS
Conceptual Problems
-
Arrange sp , sp 3 , and sp 2 in order of increasing strength of the bond formed to a hydrogen atom. Explain your reasoning.
-
What atomic orbitals are combined to form sp 3 , sp , sp 3 d 2 , and sp 3 d ? What is the maximum number of electron-pair bonds that can be formed using each set of hybrid orbitals?
-
Why is it incorrect to say that an atom with sp 2 hybridization will form only three bonds? The carbon atom in the carbonate anion is sp 2 hybridized. How many bonds to carbon are present in the carbonate ion? Which orbitals on carbon are used to form each bond?
-
If hybridization did not occur, how many bonds would N, O, C, and B form in a neutral molecule, and what would be the approximate molecular geometry?
-
How are hybridization and molecular geometry related? Which has a stronger correlation—molecular geometry and hybridization or Lewis structures and hybridization?
-
In the valence bond approach to bonding in BeF 2 , which step(s) require(s) an energy input, and which release(s) energy?
-
The energies of hybrid orbitals are intermediate between the energies of the atomic orbitals from which they are formed. ¿Por qué?
-
How are lone pairs on the central atom treated using hybrid orbitals?
-
Because nitrogen bonds to only three hydrogen atoms in ammonia, why doesn’t the nitrogen atom use sp 2 hybrid orbitals instead of sp 3 hybrids?
-
Using arguments based on orbital hybridization, explain why the CCl 6 2− ion does not exist.
-
Species such as NF 5 2− and OF 4 2− are unknown. If 3 d atomic orbitals were much lower energy, low enough to be involved in hybrid orbital formation, what effect would this have on the stability of such species? ¿Por qué? What molecular geometry, electron-pair geometry, and hybridization would be expected for each molecule?
Numerical Problems
-
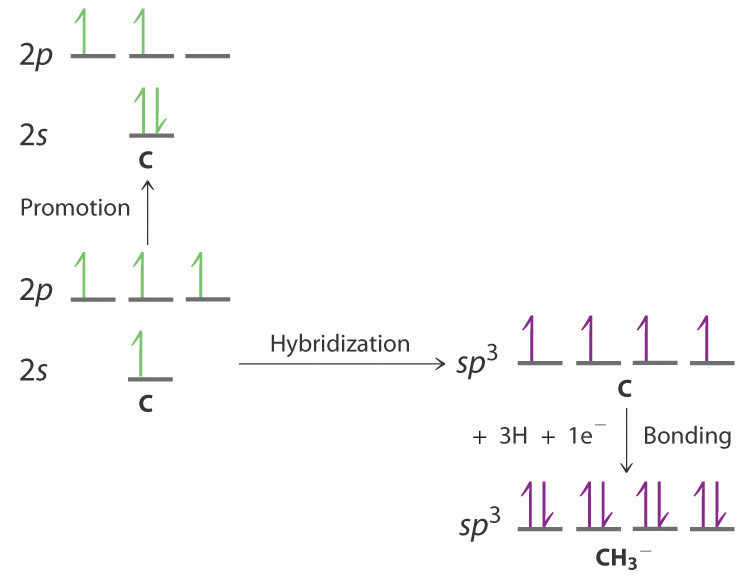
Draw an energy-level diagram showing promotion and hybridization to describe the bonding in CH 3 − . How does your diagram compare with that for methane? What is the molecular geometry?
-
Draw an energy-level diagram showing promotion and hybridization to describe the bonding in CH 3 + . How does your diagram compare with that for methane? What is the molecular geometry?
-
Draw the molecular structure, including any lone pairs on the central atom, state the hybridization of the central atom, and determine the molecular geometry for each molecule.
- BBr 3
- PCl 3
- NO 3 −
-
Draw the molecular structure, including any lone pairs on the central atom, state the hybridization of the central atom, and determine the molecular geometry for each species.
- AsBr 3
- CF 3 +
- H 2 O
-
What is the hybridization of the central atom in each of the following?
- CF 4
- CCl 2 2−
- IO 3 −
- SiH 4
-
What is the hybridization of the central atom in each of the following?
- CCl 3 +
- CBr 2 O
- CO 3 2−
- IBr 2 −
-
What is the hybridization of the central atom in PF 6 − ? Is this ion likely to exist? Why or why not? What would be the shape of the molecule?
-
What is the hybridization of the central atom in SF 5 − ? Is this ion likely to exist? Why or why not? What would be the shape of the molecule?
Numerical Answers
-
The promotion and hybridization process is exactly the same as shown for CH 4 in the chapter. The only difference is that the C atom uses the four singly occupied sp 3 hybrid orbitals to form electron-pair bonds with only three H atoms, and an electron is added to the fourth hybrid orbital to give a charge of 1–. The electron-pair geometry is tetrahedral, but the molecular geometry is pyramidal, as in NH 3 .
-
-
-
sp 2 , trigonal planar
-
sp 3 , pyramidal
-
sp 2 , trigonal planar
-
-
-
The central atoms in CF 4 , CCl 2 2– , IO 3 − , and SiH 4 are all sp 3 hybridized.
-
-
The phosphorus atom in the PF 6 − ion is sp 3 d 2 hybridized, and the ion is octahedral. The PF 6 − ion is isoelectronic with SF 6 and has essentially the same structure. It should therefore be a stable species.
9.6: MULTIPLE BONDS
Conceptual Problems
- What information is obtained by using the molecular orbital approach to bonding in O 3 that is not obtained using the VSEPR model? Can this information be obtained using a Lewis electron-pair approach?
- How is resonance explained using the molecular orbital approach?
- Indicate what information can be obtained by each method:
| Lewis Electron Structures | VSEPR Model | Valence Bond Theory | Molecular Orbital Theory | |
|---|---|---|---|---|
| Geometry | ||||
| Resonance | ||||
| Orbital Hybridization | ||||
| Reactivity | ||||
| Expanded Valences | ||||
| Bond Order |
Numerical Problems
- Using both a hybrid atomic orbital and molecular orbital approaches, describe the bonding in (BCl_3) and (CS_3^{2−}).
- Use both a hybrid atomic orbital and molecular orbital approaches to describe the bonding in (CO_2) and (N_3^−).
9.7: MOLECULAR ORBITALS
9.8: SECOND-ROW DIATOMIC MOLECULES