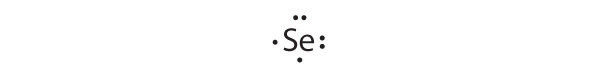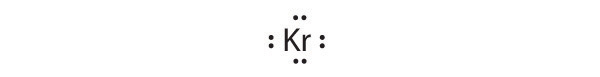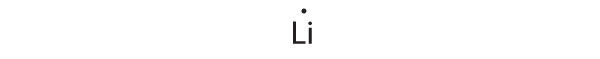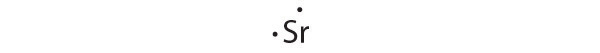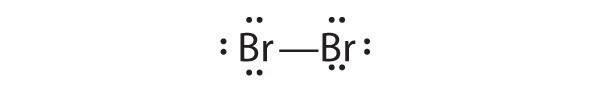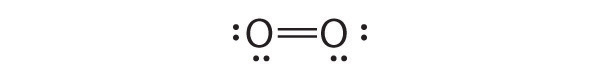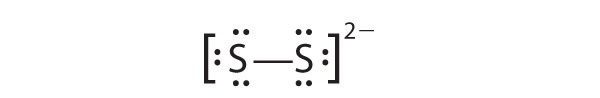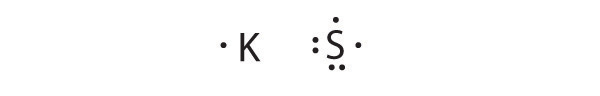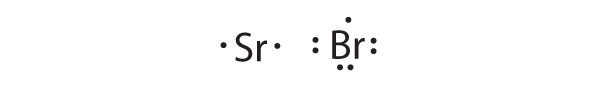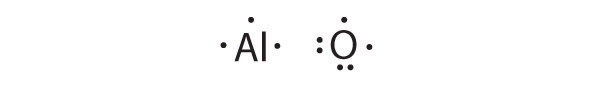Estos son ejercicios de tarea para acompañar el Mapa de texto creado para “Química: la ciencia central” por Brown et al. Se pueden encontrar bancos de preguntas de química general complementaria para otros mapas de texto y se puede acceder aquí . Además de estas preguntas disponibles públicamente, el acceso al banco privado de problemas para su uso en exámenes y tareas está disponible para los profesores solo de manera individual; comuníquese con Delmar Larsen para obtener una cuenta con permiso de acceso.
8.1: BONOS QUÍMICOS, SÍMBOLOS DE LEWIS Y LA REGLA DE OCTET
Problemas conceptuales
- El sistema de electrones de Lewis es un enfoque simplificado para comprender la unión en compuestos covalentes e iónicos. ¿Por qué los químicos todavía lo encuentran útil?
- ¿Es un símbolo de punto de Lewis una representación exacta de los electrones de valencia en un átomo o ion? Explica tu respuesta.
- ¿Cómo puede ayudar el sistema de puntos electrónicos de Lewis a predecir la estequiometría de un compuesto y sus propiedades químicas y físicas?
- ¿Cómo es consistente un símbolo de punto de Lewis con el modelo mecánico cuántico del átomo? ¿Cómo es diferente?
Respuesta conceptual
8.2: VINCULACIÓN IÓNICA
Problemas conceptuales
-
Describa las diferencias de comportamiento entre NaOH y CH 3 OH en solución acuosa. ¿Qué solución sería un mejor conductor de electricidad? Explica tu razonamiento.
-
¿Cuál es la relación entre la fuerza de la atracción electrostática entre iones con carga opuesta y la distancia entre los iones? ¿Cómo cambia la fuerza de las interacciones electrostáticas a medida que aumenta el tamaño de los iones?
-
¿Cuál resultará en la liberación de más energía: la interacción de un ion de sodio gaseoso con un ion de óxido gaseoso o la interacción de un ion de sodio gaseoso con un ion de bromuro gaseoso? ¿Por qué?
-
¿Qué resultará en la liberación de más energía: la interacción de un ion de cloruro gaseoso con un ion de sodio gaseoso o un ion de potasio gaseoso? Explica tu respuesta.
-
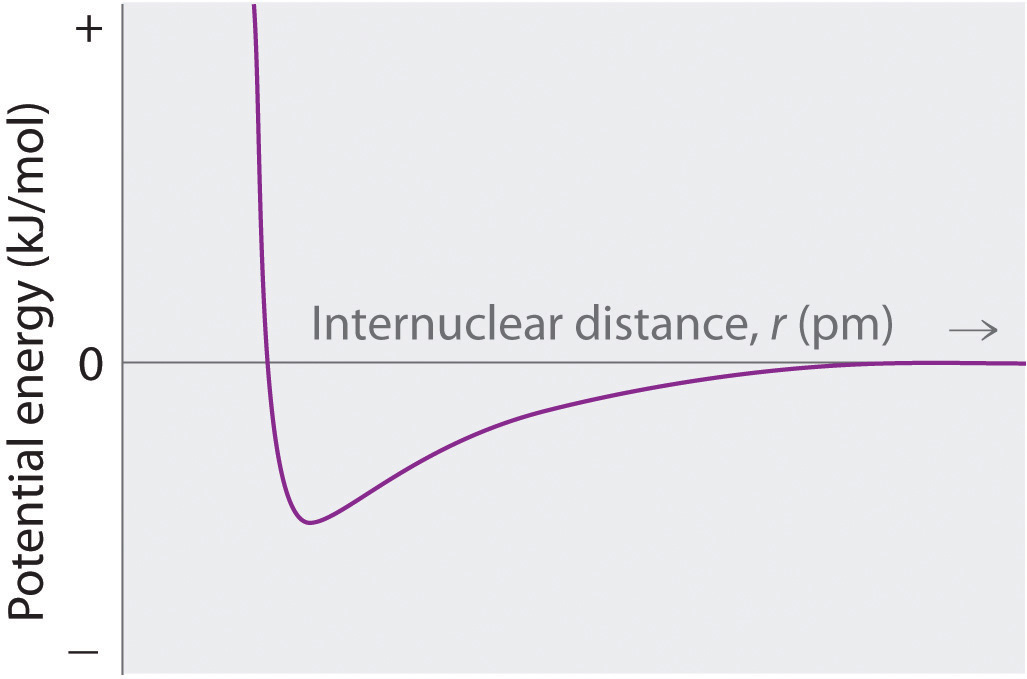
¿Cuáles son las interacciones predominantes cuando los iones con carga opuesta son
- lejos?
- a distancias internucleares cercanas a r 0 ?
- muy juntos (a una distancia que es menor que la suma de los radios iónicos)?
-
Varios factores contribuyen a la estabilidad de los compuestos iónicos. Describa un tipo de interacción que desestabilice compuestos iónicos. Describa las interacciones que estabilizan compuestos iónicos.
-
¿Cuál es la relación entre la energía electrostática atractiva entre las partículas cargadas y la distancia entre las partículas?
Respuesta conceptual
-
-
-
La interacción de un ion de sodio y un ion de óxido. La energía de atracción electrostática entre iones de carga opuesta es directamente proporcional a la carga en cada ion ( Q 1 y Q 2 en la ecuación 9.1). Por lo tanto, se libera más energía a medida que aumenta la carga en los iones (suponiendo que la distancia internuclear no aumenta sustancialmente). Un ion de sodio tiene una carga +1; un ión óxido, una carga −2; y un ion bromuro, una carga -1. Para la interacción de un ion de sodio con un ion de óxido, Q 1 = +1 y Q 2 = −2, mientras que para la interacción de un ion de sodio con un ion de bromuro, Q 1 = +1 y Q 2 = −1. El valor mayor de Q 1 × Q 2 para la interacción de iones de sodio-iones de óxido significa que liberará más energía.
Problemas numéricos
-
¿Cómo se compara la energía de la interacción electrostática entre iones con cargas +1 y −1 con la interacción entre iones con cargas +3 y −1 si la distancia entre los iones es la misma en ambos casos? ¿Cómo se compara esto con la magnitud de la interacción entre iones con cargas +3 y −3?
-
¿Cuántos gramos de MgCl gaseoso 2 se necesitan para proporcionar la misma energía electrostática atractiva que 0,5 mol de LiCl gaseoso? Los radios iónicos son Li + = 76 pm, Mg +2 = 72 pm y Cl – = 181 pm.
-
Dibuje un diagrama que muestre la relación entre la energía potencial y la distancia internuclear (de r = ∞ a r = 0) para que se forme la interacción de un ion bromuro y un ion potasio KBr gaseoso. Explique por qué la energía del sistema aumenta a medida que la distancia entre los iones disminuye de r = r 0 a r = 0. [ 19459339]
-
Calcule la magnitud de la energía electrostática de atracción ( E , en kilojulios) para 85.0 g de pares de iones SrS gaseosos. La distancia internuclear observada en la fase gaseosa es 244.05 pm.
-
What is the electrostatic attractive energy ( E , in kilojoules) for 130 g of gaseous HgI 2 ? The internuclear distance is 255.3 pm.
Numerical Answers
-
According to Equation 9.1, in the first case Q 1 Q 2 = (+1)(−1) = −1; in the second case, Q 1 Q 2 = (+3)(−1) = −3. Thus, E will be three times larger for the +3/−1 ions. For +3/−3 ions, Q 1 Q 2 = (+3)(−3) = −9, so E will be nine times larger than for the +1/−1 ions.
-
-
At r < r 0 , the energy of the system increases due to electron–electron repulsions between the overlapping electron distributions on adjacent ions. At very short internuclear distances, electrostatic repulsions between adjacent nuclei also become important.
8.3: COVALENT BONDING
Conceptual Problems
- Which would you expect to be stronger—an S–S bond or an Se–Se bond? ¿Por qué?
- Which element—nitrogen, phosphorus, or arsenic—will form the strongest multiple bond with oxygen? ¿Por qué?
- Why do multiple bonds between oxygen and period 3 elements tend to be unusually strong?
- What can bond energies tell you about reactivity?
- Bond energies are typically reported as average values for a range of bonds in a molecule rather than as specific values for a single bond? ¿Por qué?
- If the bonds in the products are weaker than those in the reactants, is a reaction exothermic or endothermic? Explain your answer.
- A student presumed that because heat was required to initiate a particular reaction, the reaction product would be stable. Instead, the product exploded. What information might have allowed the student to predict this outcome?
Numerical Problems
-
What is the bond order about the central atom(s) of hydrazine (N 2 H 4 ), nitrogen, and diimide (N 2 H 2 )? Draw Lewis electron structures for each compound and then arrange these compounds in order of increasing N–N bond distance. Which of these compounds would you expect to have the largest N–N bond energy? Explain your answer.
-
What is the carbon–carbon bond order in ethylene (C 2 H 4 ), BrH 2 CCH 2 Br, and FCCH? Arrange the compounds in order of increasing C–C bond distance. Which would you expect to have the largest C–C bond energy? ¿Por qué?
-
From each pair of elements, select the one with the greater bond strength? Explain your choice in each case.
- P–P, Sb–Sb
- Cl–Cl, I–I
- O–O, Se–Se
- S–S, Cl–Cl
- Al–Cl, B–Cl
-
From each pair of elements, select the one with the greater bond strength? Explain your choice in each case.
- Te–Te, S–S
- C–H, Ge–H
- Si–Si, P–P
- Cl–Cl, F–F
- Ga–H, Al–H
-
Approximately how much energy per mole is required to completely dissociate acetone [(CH 3 ) 2 CO] and urea [(NH 2 ) 2 CO] into their constituent atoms?
-
Approximately how much energy per mole is required to completely dissociate ethanol, formaldehyde, and hydrazine into their constituent atoms?
-
Is the reaction of diimine (N 2 H 2 ) with oxygen to produce nitrogen and water exothermic or endothermic? Quantify your answer.
Numerical Answer
-
N 2 H 4 , bond order 1; N 2 H 2 , bond order 2; N 2 , bond order 3; N–N bond distance: N 2 < N 2 H 2 < N 2 H 4 ; Largest bond energy: N 2 ; Highest bond order correlates with strongest and shortest bond.
8.4: BOND POLARITY AND ELECRONEGATIVITY
Conceptual Problems
-
Why do ionic compounds such as KI exhibit substantially less than 100% ionic character in the gas phase?
-
Of the compounds LiI and LiF, which would you expect to behave more like a classical ionic compound? Which would have the greater dipole moment in the gas phase? Explain your answers.
Numerical Problems
-
Predict whether each compound is purely covalent, purely ionic, or polar covalent.
- RbCl
- S 8
- TiCl 2
- SbCl 3
- LiI
- Br 2
-
Based on relative electronegativities, classify the bonding in each compound as ionic, covalent, or polar covalent. Indicate the direction of the bond dipole for each polar covalent bond.
- NO
- HF
- MgO
- AlCl 3
- SiO 2
- the C=O bond in acetone
- O 3
-
Based on relative electronegativities, classify the bonding in each compound as ionic, covalent, or polar covalent. Indicate the direction of the bond dipole for each polar covalent bond.
- NaBr
- OF 2
- BCl 3
- the S–S bond in CH 3 CH 2 SSCH 2 CH 3
- the C–Cl bond in CH 2 Cl 2
- the O–H bond in CH 3 OH
- PtCl 4 2−
-
Classify each species as having 0%–40% ionic character, 40%–60% ionic character, or 60%–100% ionic character based on the type of bonding you would expect. Justify your reasoning.
- CaO
- S 8
- AlBr 3
- ICl
- Na 2 S
- SiO 2
- LiBr
-
If the bond distance in HCl (dipole moment = 1.109 D) were double the actual value of 127.46 pm, what would be the effect on the charge localized on each atom? What would be the percent negative charge on Cl? At the actual bond distance, how would doubling the charge on each atom affect the dipole moment? Would this represent more ionic or covalent character?
-
Calculate the percent ionic character of HF (dipole moment = 1.826 D) if the H–F bond distance is 92 pm.
-
Calculate the percent ionic character of CO (dipole moment = 0.110 D) if the C–O distance is 113 pm.
-
Calculate the percent ionic character of PbS and PbO in the gas phase, given the following information: for PbS, r = 228.69 pm and µ = 3.59 D; for PbO, r = 192.18 pm and µ = 4.64 D. Would you classify these compounds as having covalent or polar covalent bonds in the solid state?
8.5: DRAWING LEWIS STRUCTURES
Conceptual Problems
-
Compare and contrast covalent and ionic compounds with regard to
- volatility.
- melting point.
- electrical conductivity.
- physical appearance.
-
What are the similarities between plots of the overall energy versus internuclear distance for an ionic compound and a covalent compound? Why are the plots so similar?
-
Which atom do you expect to be the central atom in each of the following species?
- SO 4 2−
- NH 4 +
- BCl 3
- SO 2 Cl 2 ?
-
Which atom is the central atom in each of the following species?
- PCl 3
- CHCl 3
- SO 2
- IF 3 ?
-
What is the relationship between the number of bonds typically formed by the period 2 elements in groups 14, 15, and 16 and their Lewis electron structures?
-
Although formal charges do not represent actual charges on atoms in molecules or ions, they are still useful. ¿Por qué?
Numerical Problems
-
Give the electron configuration and the Lewis dot symbol for the following. How many more electrons can each atom accommodate?
- Se
- Kr
- Li
- Sr
- H
-
Give the electron configuration and the Lewis dot symbol for the following. How many more electrons can each atom accommodate?
- Na
- Br
- Ne
- C
- Ga
-
Based on Lewis dot symbols, predict the preferred oxidation state of Be, F, B, and Cs.
-
Based on Lewis dot symbols, predict the preferred oxidation state of Br, Rb, O, Si, and Sr.
-
Based on Lewis dot symbols, predict how many bonds gallium, silicon, and selenium will form in their neutral compounds.
-
Determine the total number of valence electrons in the following.
- Cr
- Cu +
- NO +
- XeF 2
- Br 2
- CH 2 Cl 2
- NO 3 −
- H 3 O +
-
Determine the total number of valence electrons in the following.
- Ag
- Pt 2+
- H 2 S
- OH −
- I 2
- CH 4
- SO 4 2−
- NH 4 + .
-
Draw Lewis electron structures for the following.
- F 2
- SO 2
- AlCl 4 −
- SO 3 2−
- BrCl
- XeF 4
- NO +
- PCl 3
-
Draw Lewis electron structures for the following.
- Br 2
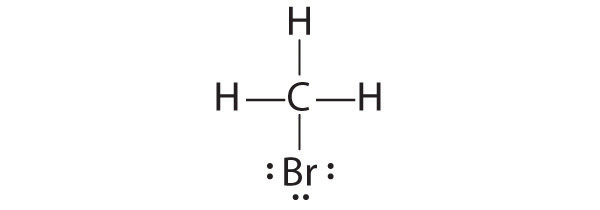
- CH 3 Br
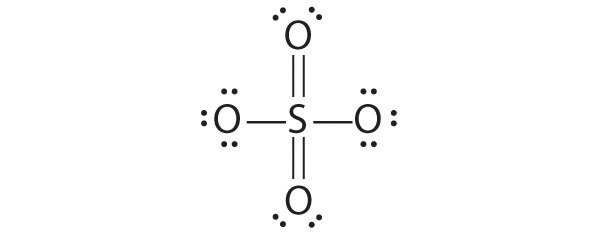
- SO 4 2−
- O 2
- S 2 2−
- BF 3
-
Draw Lewis electron structures for CO 2 , NO 2 − , SO 2 , and NO 2 + . From your diagram, predict which pair(s) of compounds have similar electronic structures.
-
Write Lewis dot symbols for each pair of elements. For a reaction between each pair of elements, predict which element is the oxidant, which element is the reductant, and the final stoichiometry of the compound formed.
- K, S
- Sr, Br
- Al, O
- Mg, Cl
-
Write Lewis dot symbols for each pair of elements. For a reaction between each pair of elements, predict which element is the oxidant, which element is the reductant, and the final stoichiometry of the compound formed.
- Li, F
- Cs, Br
- Ca, Cl
- B, F
-
Use Lewis dot symbols to predict whether ICl and NO 4 − are chemically reasonable formulas.
-
Draw a plausible Lewis electron structure for a compound with the molecular formula Cl 3 PO.
-
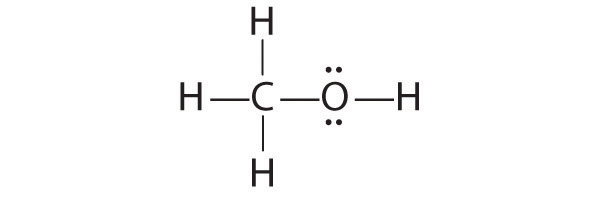
Draw a plausible Lewis electron structure for a compound with the molecular formula CH 4 O.
-
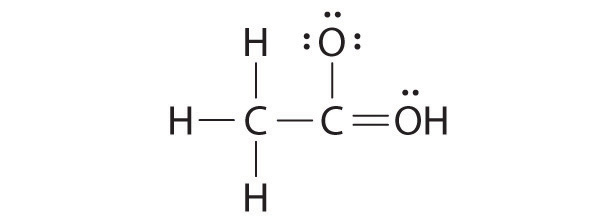
While reviewing her notes, a student noticed that she had drawn the following structure in her notebook for acetic acid:
Why is this structure not feasible? Draw an acceptable Lewis structure for acetic acid. Show the formal charges of all nonhydrogen atoms in both the correct and incorrect structures.
-
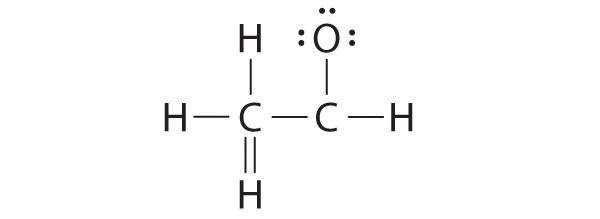
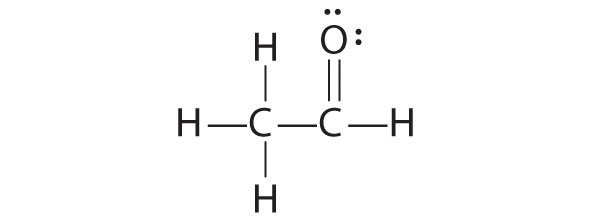
A student proposed the following Lewis structure shown for acetaldehyde.
Why is this structure not feasible? Draw an acceptable Lewis structure for acetaldehyde. Show the formal charges of all nonhydrogen atoms in both the correct and incorrect structures.
-
Draw the most likely structure for HCN based on formal charges, showing the formal charge on each atom in your structure. Does this compound have any plausible resonance structures? If so, draw one.
-
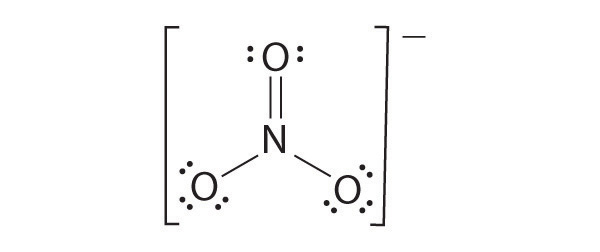
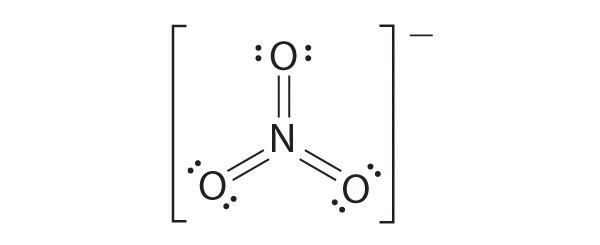
Draw the most plausible Lewis structure for NO 3 − . Does this ion have any other resonance structures? Draw at least one other Lewis structure for the nitrate ion that is not plausible based on formal charges.
-
At least two Lewis structures can be drawn for BCl 3 . Using arguments based on formal charges, explain why the most likely structure is the one with three B–Cl single bonds.
-
Using arguments based on formal charges, explain why the most feasible Lewis structure for SO 4 2− has two sulfur–oxygen double bonds.
-
At least two distinct Lewis structures can be drawn for N 3 − . Use arguments based on formal charges to explain why the most likely structure contains a nitrogen–nitrogen double bond.
-
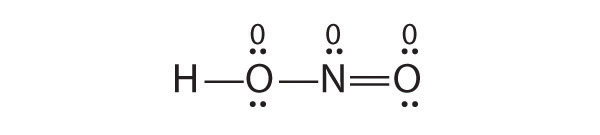
Is H–O–N=O a reasonable structure for the compound HNO 2 ? Justify your answer using Lewis electron dot structures.
-
Is H–O=C–H a reasonable structure for a compound with the formula CH 2 O? Use Lewis electron dot structures to justify your answer.
-
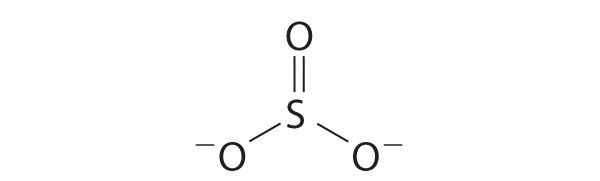
Explain why the following Lewis structure for SO 3 2− is or is not reasonable.
Numerical Answers
-
-
[Ar]4 s 2 3 d 10 4 p 4
Selenium can accommodate two more electrons, giving the Se 2− ion.
-
[Ar]4 s 2 3 d 10 4 p 6
Krypton has a closed shell electron configuration, so it cannot accommodate any additional electrons.
-
1 s 2 2 s 1
Lithium can accommodate one additional electron in its 2 s orbital, giving the Li − ion.
-
[Kr]5 s 2
Strontium has a filled 5 s subshell, and additional electrons would have to be placed in an orbital with a higher energy. Thus strontium has no tendency to accept an additional electron.
-
1 s 1
Hydrogen can accommodate one additional electron in its 1 s orbital, giving the H − ion.
-
-
-
Be 2+ , F − , B 3+ , Cs +
-
-
-
-
- 11
- 8
- 8
- 8
- 14
- 8
- 32
- 8
-
-
-
-
-
-
K is the reductant; S is the oxidant. The final stoichiometry is K 2 S.
-
Sr is the reductant; Br is the oxidant. The final stoichiometry is SrBr 2 .
-
Al is the reductant; O is the oxidant. The final stoichiometry is Al 2 O 3 .
-
Mg is the reductant; Cl is the oxidant. The final stoichiometry is MgCl 2 .
-
-
-
-
-
The only structure that gives both oxygen and carbon an octet of electrons is the following:
-
-
The student’s proposed structure has two flaws: the hydrogen atom with the double bond has four valence electrons (H can only accommodate two electrons), and the carbon bound to oxygen only has six valence electrons (it should have an octet). An acceptable Lewis structure is
The formal charges on the correct and incorrect structures are as follows:
-
-
The most plausible Lewis structure for NO 3 − is:
There are three equivalent resonance structures for nitrate (only one is shown), in which nitrogen is doubly bonded to one of the three oxygens. In each resonance structure, the formal charge of N is +1; for each singly bonded O, it is −1; and for the doubly bonded oxygen, it is 0.
The following is an example of a Lewis structure that is not plausible:
This structure nitrogen has six bonds (nitrogen can form only four bonds) and a formal charge of –1.
-
-
With four S–O single bonds, each oxygen in SO 4 2− has a formal charge of −1, and the central sulfur has a formal charge of +2. With two S=O double bonds, only two oxygens have a formal charge of –1, and sulfur has a formal charge of zero. Lewis structures that minimize formal charges tend to be lowest in energy, making the Lewis structure with two S=O double bonds the most probable.
-
-
Yes. This is a reasonable Lewis structure, because the formal charge on all atoms is zero, and each atom (except H) has an octet of electrons.
8.6: RESONANCE STRUCTURES
Conceptual Problems
- Why are resonance structures important?
- In what types of compounds are resonance structures particularly common?
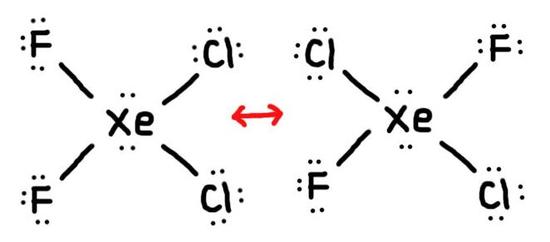
- True or False, The picture below is a resonance structure?
Numerical Problems
- Draw all the resonance structures for each ion.
- HSO 4 −
- HSO 3 −
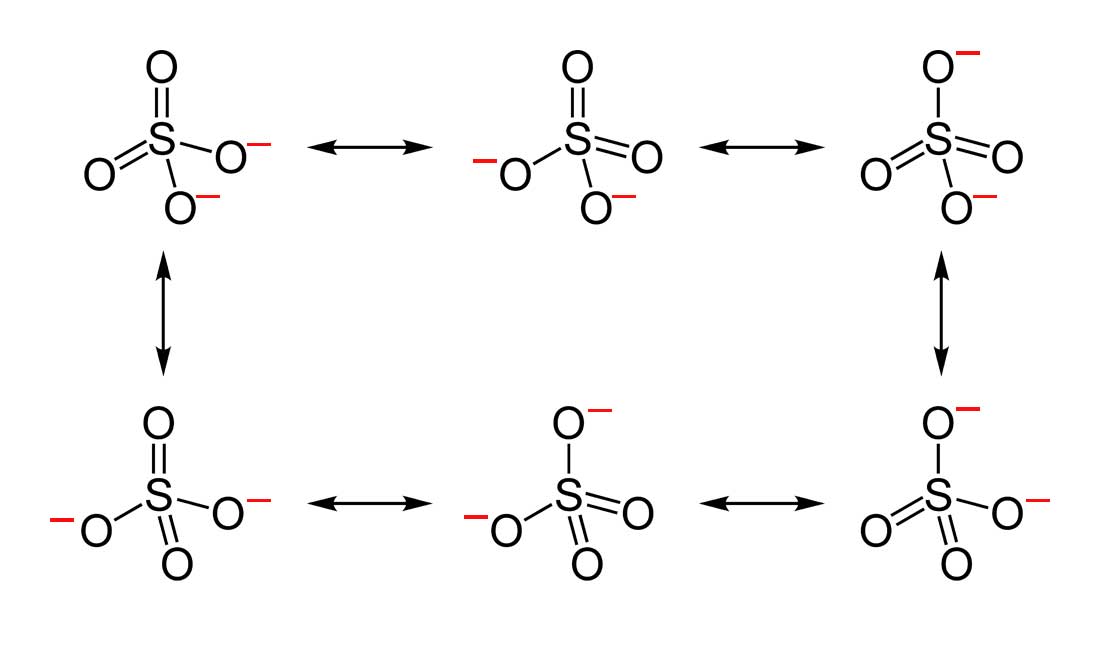
- Draw the Lewis Dot Structure for SO 4 2 – and all possible resonance structures. Which of the following resonance structure is not favored among the Lewis Structures? Explain why. Assign Formal Charges.
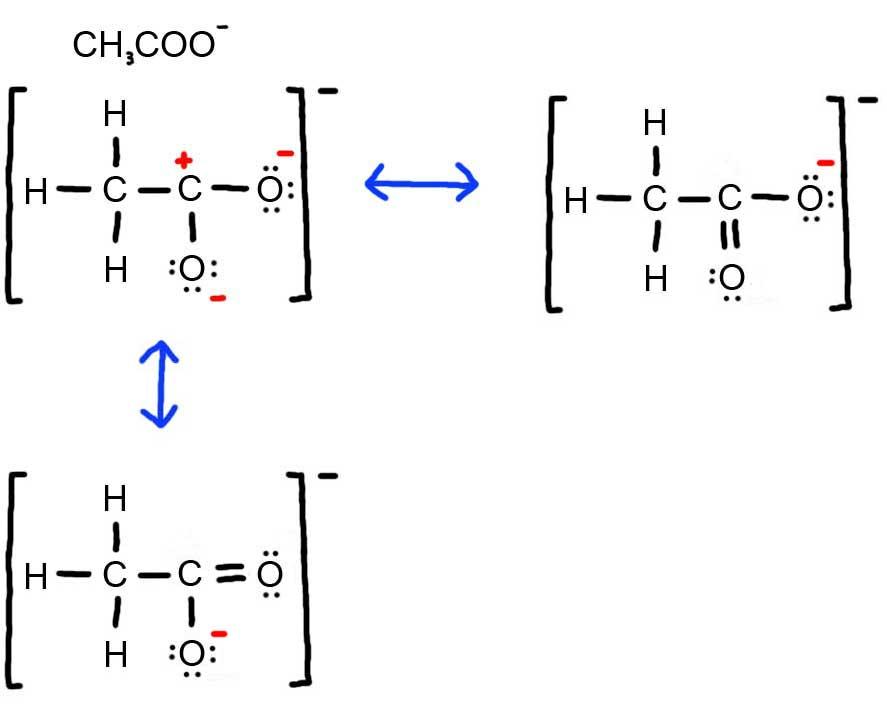
- Draw the Lewis Dot Structure for CH 3 COO – and all possible resonance structures. Assign Formal Charges. Choose the most favorable Lewis Structure.
- Draw the Lewis Dot Structure for H PO 3 2 – and all possible resonance structures. Assign Formal Charges.
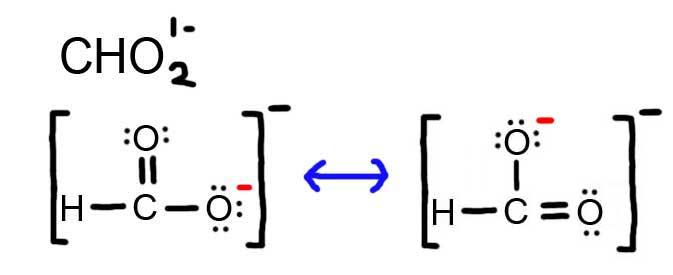
- Draw the Lewis Dot Structure for CHO 2 1 – and all possible resonance structures. Assign Formal Charges.
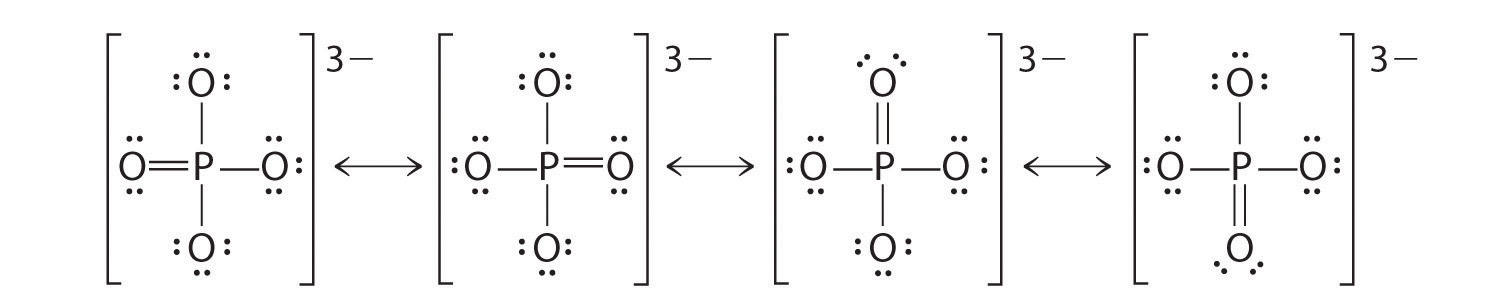
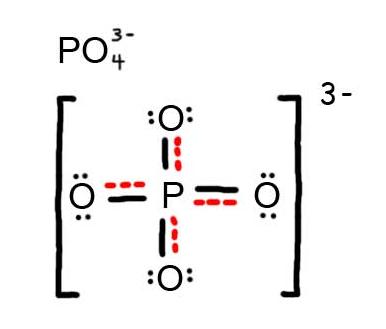
- Draw the Resonance Hybrid Structure for P O 4 3 – .
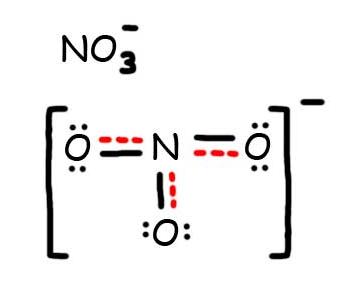
- Draw the Resonance Hybrid Structure for N O 3 – .
Numerical Answers
2. False, because the electrons were not moved around, only the atoms (this violates the Resonance Structure Rules).
3. Below are the all Lewis dot structure with formal charges (in red) for Sulfate ( SO 4 2 – ). There isn’t a most favorable resonance of the Sulfate ion because they are all identical in charge and there is no change in Electronegativity between the Oxygen atoms.
4. Below is the resonance for CH 3 COO – , formal charges are displayed in red. The Lewis Structure with the most formal charges is not desirable, because we want the Lewis Structure with the least formal charge.
5. The resonance for HPO 3 2 – , and the formal charges (in red).
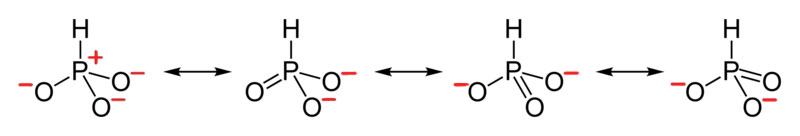
6. The resonance for CHO 2 1 – , and the formal charges (in red).
7. The resonance hybrid for PO 4 3 – , hybrid bonds are in red.
8. The resonance hybrid for NO 3 – , hybrid bonds are in red.
8.7: EXCEPTIONS TO THE OCTET RULE
Conceptual Problems
- What regions of the periodic table contain elements that frequently form molecules with an odd number of electrons? Explain your answer.
- How can atoms expand their valence shell? What is the relationship between an expanded valence shell and the stability of an ion or a molecule?
- What elements are known to form compounds with less than an octet of electrons? Why do electron-deficient compounds form?
- List three elements that form compounds that do not obey the octet rule. Describe the factors that are responsible for the stability of these compounds.
Numerical Problems
-
What is the major weakness of the Lewis system in predicting the electron structures of PCl 6 − and other species containing atoms from period 3 and beyond?
-
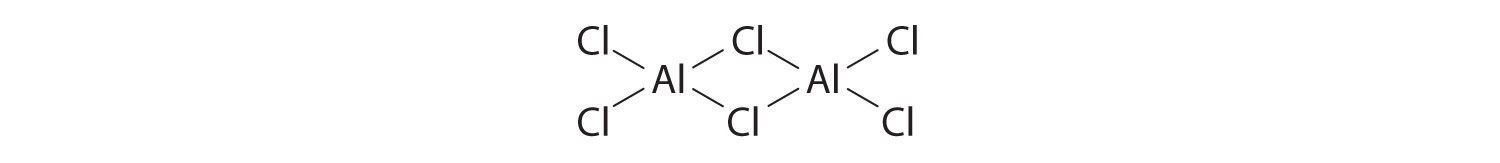
The compound aluminum trichloride consists of Al 2 Cl 6 molecules with the following structure (lone pairs of electrons removed for clarity):
Does this structure satisfy the octet rule? What is the formal charge on each atom? Given the chemical similarity between aluminum and boron, what is a plausible explanation for the fact that aluminum trichloride forms a dimeric structure rather than the monomeric trigonal planar structure of BCl 3 ?
-
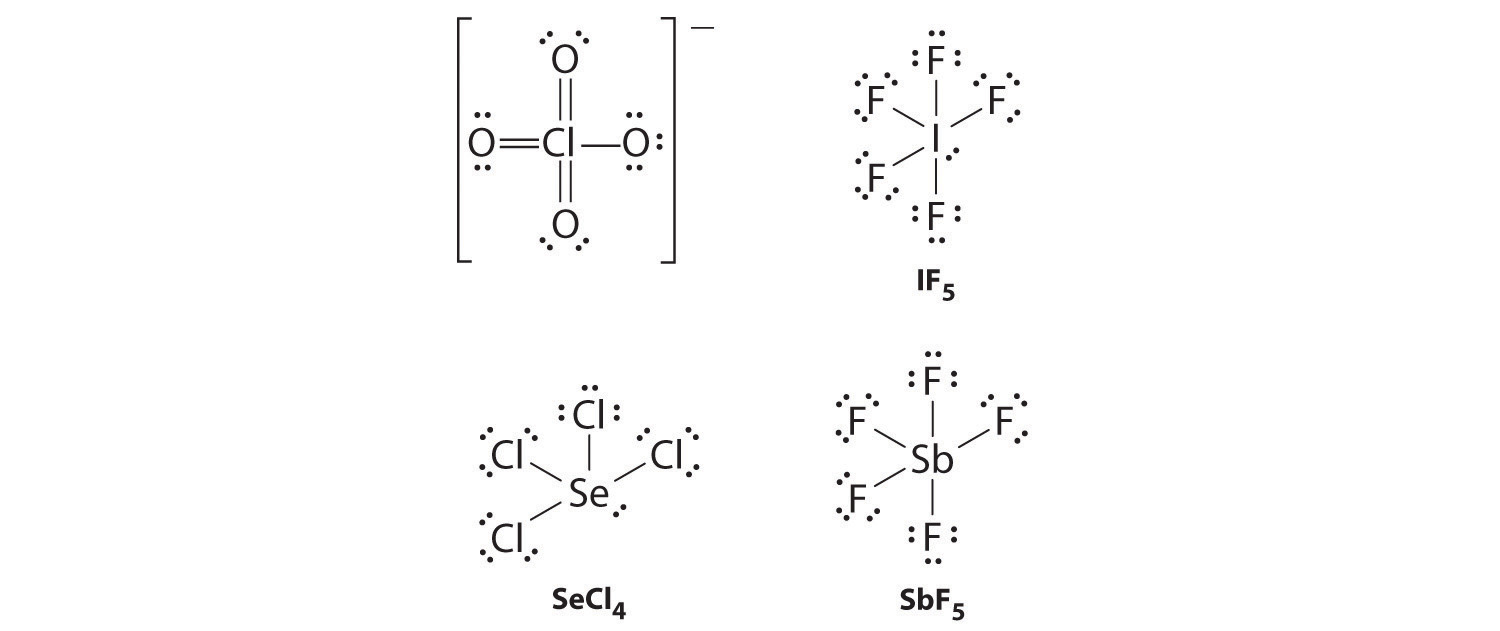
Draw Lewis electron structures for ClO 4 − , IF 5 , SeCl 4 , and SbF 5 .
-
Draw Lewis electron structures for ICl 3 , Cl 3 PO, Cl 2 SO, and AsF 6 − .
-
Draw plausible Lewis structures for the phosphate ion, including resonance structures. What is the formal charge on each atom in your structures?
-
Draw an acceptable Lewis structure for PCl 5 , a compound used in manufacturing a form of cellulose. What is the formal charge of the central atom? What is the oxidation number of the central atom?
-
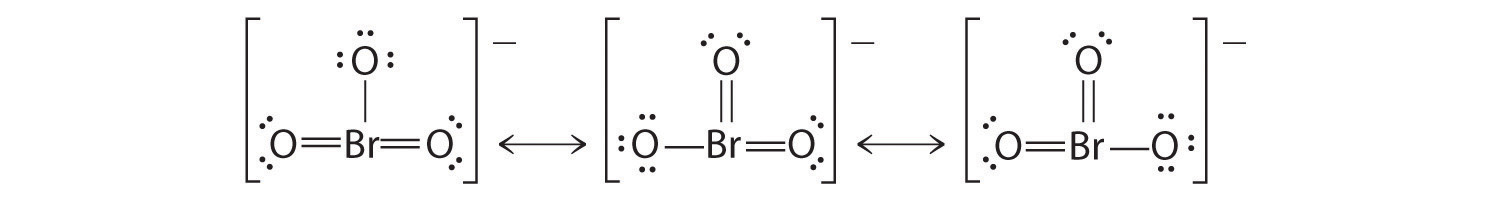
Using Lewis structures, draw all of the resonance structures for the BrO 3 − ion.
-
Draw an acceptable Lewis structure for xenon trioxide (XeO 3 ), including all resonance structures.
Numerical Answers
-
-
-
ClO 4 − (one of four equivalent resonance structures)
-
-
The formal charge on phosphorus is 0, while three oxygen atoms have a formal charge of −1 and one has a formal charge of zero.
-
Practice Problems
- Draw the Lewis structure for the molecule I 3 – .
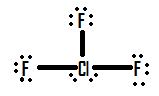
- Draw the molecule ClF 3 .
- The central atom for an expanded octet must have an atomic number larger than what?
- Draw the Lewis structure for the molecule NO 2 .
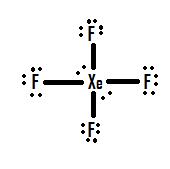
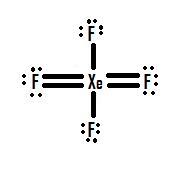
- Which Lewis structure is more likely?
Practice Answers
1. 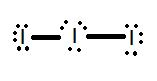
2. 
3. 10 (Sodium and higher)
4. 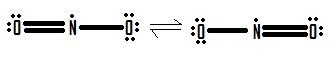
5. 
8.8: STRENGTHS OF COVALENT BONDS