Estos son ejercicios de tarea para acompañar el Mapa de texto creado para “Química: la ciencia central” por Brown et al. Se pueden encontrar bancos de preguntas de química general complementaria para otros mapas de texto y se puede acceder aquí . Además de estas preguntas disponibles públicamente, el acceso al banco privado de problemas para su uso en exámenes y tareas está disponible para los profesores solo de manera individual; comuníquese con Delmar Larsen para obtener una cuenta con permiso de acceso.
5.1: La naturaleza de la energía
Problemas conceptuales
-
¿Cuál es la relación entre el trabajo mecánico y la energía?
-
¿Trabaja una persona con una masa de 50 kg que sube una altura de 15 m? Explica tu respuesta. ¿Esa misma persona trabaja mientras desciende una montaña?
-
Si una persona ejerce una fuerza sobre un objeto inamovible, ¿trabaja esa persona? Explica tu respuesta.
-
Explica las diferencias entre la energía eléctrica, la energía nuclear y la energía química.
-
El capítulo describe la energía térmica, la energía radiante, la energía eléctrica, la energía nuclear y la energía química. ¿Qué forma (s) de energía están representadas por cada uno de los siguientes?
- luz solar
- la energía producida por un tubo de rayos catódicos, como el que se encuentra en un televisor
- la energía emitida por la radiactividad
- la energía emitida por una vela encendida
- la energía asociada con una máquina de vapor
- la energía emitida por un teléfono celular
- la energía asociada con una barra de dinamita
-
Describa las diversas formas de energía que se interconvierten cuando se enciende una linterna.
-
Describe las formas de energía que se interconvierten cuando despega el transbordador espacial.
-
Clasifique cada uno de los siguientes como representación de energía cinética o energía potencial.
- la energía asociada con una computadora portátil sentada en el borde de un escritorio
- palear nieve
- agua que sale de una boca de incendios
- la energía liberada por un terremoto
- la energía en un volcán a punto de entrar en erupción
- la energía asociada con un resorte en espiral
-
¿Las unidades de energía potencial son las mismas que las unidades de energía cinética? ¿Se puede obtener un valor absoluto para la energía potencial? Explica tu respuesta.
-
Clasifique cada uno de los siguientes como representación de energía cinética o energía potencial.
- agua en cascada sobre las Cataratas del Niágara
- un vaso de precipitados equilibrado en el borde de un fregadero
- la energía liberada durante un deslizamiento de tierra
- patinar
- la energía en un bloque de hielo en una azotea antes de un deshielo
-
¿Por qué martillar una pieza de chapa metálica hace que el metal se caliente?
Respuestas conceptuales
3. Técnicamente, la persona no está haciendo ningún trabajo, ya que el objeto no se mueve.
11. La energía cinética del martillo se transfiere al metal.
Problemas numéricos
Asegúrese de estar familiarizado con los temas discutidos en Habilidades esenciales 4 antes de continuar con los Problemas numéricos.
-
Describa la relación matemática entre (a) la energía térmica almacenada en un objeto y la masa de ese objeto y (b) la energía térmica almacenada en un objeto y la temperatura de ese objeto.
-
¿Cuánta energía (en kilojulios) se libera o almacena cuando ocurre lo siguiente?
- Un jugador de fútbol de 230 lb se levanta a una altura de 4.00 pies
- Un gato de 11.8 lb salta desde una altura de 6.50 pies
- Un libro de 3.75 lb se cae de un estante que tiene 5.50 pies de altura.
-
Calcule cuánta energía (en kilojulios) se libera o almacena cuando ocurre cada uno de los siguientes:
- Un patinador de hielo de 130 lb se levanta 7.50 pies del hielo.
- Un niño de 48 lb salta desde una altura de 4.0 pies
- Una lámpara de 18.5 lb cae desde un techo de 10.0 pies.
-
Un automóvil que pesa 1438 kg se cae de un puente que tiene 211 pies de altura. Ignorando la resistencia del aire, ¿cuánta energía se libera cuando el automóvil golpea el agua?
-
Una montaña rusa de 1 tn llena de pasajeros alcanza una altura de 28 m antes de acelerar cuesta abajo. ¿Cuánta energía se libera cuando la montaña rusa llega al pie de la colina? Suponga que no se pierde energía debido a la fricción.
Respuestas numéricas
1.
- El contenido de energía térmica de un objeto es directamente proporcional a su masa.
- El contenido de energía térmica de un objeto es directamente proporcional a su temperatura.
- 1,3 kJ almacenados
- 0.26 kJ lanzado
- 0.251 kJ lanzado
5.
-
250 kJ lanzado
5.2: La primera ley de la termodinámica
Problemas conceptuales
- Describe cómo un péndulo oscilante que se ralentiza con el tiempo ilustra la primera ley de la termodinámica.
- Cuando se bombea aire al neumático de una bicicleta, el aire se comprime. Assuming that the volume is constant, express the change in internal energy in terms of q and w.
- What is the relationship between enthalpy and internal energy for a reaction that occurs at constant pressure?
- An intrepid scientist placed an unknown salt in a small amount of water. All the salt dissolved in the water, and the temperature of the solution dropped several degrees.
- What is the sign of the enthalpy change for this reaction?
- Assuming the heat capacity of the solution is the same as that of pure water, how would the scientist calculate the molar enthalpy change?
- Propose an explanation for the decrease in temperature.
- For years, chemists and physicists focused on enthalpy changes as a way to measure the spontaneity of a reaction. What arguments would you use to convince them not to use this method?
- What is the relationship between enthalpy and internal energy for a reaction that occurs at constant volume?
- The enthalpy of combustion (ΔH comb ) is defined thermodynamically as the enthalpy change for complete oxidation. The complete oxidation of hydrocarbons is represented by the following general equation: hydrocarbon + O 2 (g) → CO 2 (g) + H 2 O(g). Enthalpies of combustion from reactions like this one can be measured experimentally with a high degree of precision. It has been found that the less stable the reactant, the more heat is evolved, so the more negative the value of ΔHcomb. In each pair of hydrocarbons, which member do you expect to have the greater (more negative) heat of combustion? Justify your answers.
- cyclopropane or cyclopentane
- butane or 2-methylpropane
- hexane or cyclohexane
- Using a structural argument, explain why the trans isomer of 2-butene is more stable than the cis isomer. The enthalpies of formation of cis- and trans-2-butene are −7.1 kJ/mol and −11.4 kJ/mol, respectively.
- Using structural arguments, explain why cyclopropane has a positive
ΔH f ° (12.7 kJ/mol), whereas cyclopentane has a negativeΔH f° (−18.4 kJ/mol). (Hint: consider bond angles.)
Conceptual Answers
- At constant pressure, ΔH = ΔE + PΔV.
-
In some simple case, when a reaction only involves solids, liquids or liquid solution, we can say that (ΔH=ΔE). When a reaction involves gases, we can say that (ΔE = ΔH − RTΔn). However, if we have a complicated case, it is hard to use (ΔH) to measure the spontaneity of a reaction. For example, when a reaction involved gases, liquid and solid, it is really hard for us to defined (Δ(PV)).
- With bond angles of 60°, cyclopropane is highly strained, causing it to be less stable than cyclopentane, which has nearly ideal tetrahedral geometry at each carbon atom.
Numerical Problems
- A block of CO 2 weighing 15 g evaporates in a 5.0 L container at 25°C. How much work has been done if the gas is allowed to expand against an external pressure of 0.98 atm under isothermal conditions? The enthalpy of sublimation of CO 2 is 25.1 kJ/mol. What is the change in internal energy (kJ/mol) for the sublimation of CO 2 under these conditions?
- Zinc and HCl react according to the following equation:
[Zn_{(s)} + 2HCl_{(aq)} rightarrow Zn^{2+}_{(aq)} + 2Cl^−_{(aq)} + H_2(g)]
When 3.00 g of zinc metal is added to a dilute HCl solution at 1.00 atm and 25°C, and this reaction is allowed to go to completion at constant pressure, 6.99 kJ of heat must be removed to return the final solution to its original temperature. What are the values of q and w, and what is the change in internal energy?
- Acetylene torches, used industrially to cut and weld metals, reach flame temperatures as high as 3000°C. The combustion reaction is as follows:
Calculate the amount of work done against a pressure of 1.0 atm when 4.0 mol of acetylene are allowed to react with 10 mol of O 2 at 1.0 atm at 20°C. What is the change in internal energy for the reaction?
- When iron dissolves in 1.00 M aqueous HCl, the products are FeCl 2 (aq) and hydrogen gas. Calculate the work done if 30 g of Fe react with excess hydrochloric acid in a closed vessel at 20°C. How much work is done if the reaction takes place in an open vessel with an external pressure of 1.0 atm?
Numerical Answers
- −350 J; 8.2 kJ
5.3: Enthalpy
5.4: Enthalpy of Reaction
Conceptual Problems
Please be sure you are familiar with the topics discussed in Essential Skills 4 ( Section 5.6 “Essential Skills 4” ) before proceeding to the Conceptual Problems.
-
Heat implies the flow of energy from one object to another. Describe the energy flow in an
a. exothermic reaction.
b. endothermic reaction.
-
When a thermometer is suspended in an insulated thermos that contains a block of ice, the temperature recorded on the thermometer drops. Describe the direction of heat flow.
-
In each scenario, the system is defined as the mixture of chemical substances that undergoes a reaction. State whether each process is endothermic or exothermic.
- Water is added to sodium hydroxide pellets, and the flask becomes hot.
- The body metabolizes glucose, producing carbon dioxide and water.
- Ammonium nitrate crystals are dissolved in water, causing the solution to become cool.
-
In each scenario, the system is defined as the mixture of chemical substances that undergoes a reaction. Determine whether each process is endothermic or exothermic.
- Concentrated acid is added to water in a flask, and the flask becomes warm.
- Water evaporates from your skin, causing you to shiver.
- A container of ammonium nitrate detonates.
-
Is Earth’s environment an isolated system, an open system, or a closed system? Explain your answer.
-
Why is it impossible to measure the absolute magnitude of the enthalpy of an object or a compound?
-
Determine whether energy is consumed or released in each scenario. Explain your reasoning.
- A leaf falls from a tree.
- A motorboat maneuvers against a current.
- A child jumps rope.
- Dynamite detonates.
- A jogger sprints down a hill.
-
The chapter states that enthalpy is an extensive property. ¿Por qué? Describe a situation that illustrates this fact.
-
The enthalpy of a system is affected by the physical states of the reactants and the products. Explain why.
-
Is the distance a person travels on a trip a state function? Why or why not?
5.5: Calorimetry
Conceptual Problems
- Can an object have a negative heat capacity? Why or why not?
- What two factors determine the heat capacity of an object? Does the specific heat also depend on these two factors? Explain your answer.
- Explain why regions along seacoasts have a more moderate climate than inland regions do.
- Although soapstone is more expensive than brick, soapstone is frequently the building material of choice for fireplaces, particularly in northern climates with harsh winters. Propose an explanation for this.
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 4 (Section 9.9) before proceeding to the Numerical Problems.
-
Using Equation 9.6.2 and Equation 9.6.3, d erive a mathematical relationship between C s and C p .
-
Complete the following table for 28.0 g of each element at an initial temperature of 22.0°C.
Element q (J) C p [J/(mol·K)] Final T (°C) nickel 137 26.07 silicon 19.789 3.0 zinc 603 77.5 mercury 137 57 -
Using Table 9.6.1, how much heat is needed to raise the temperature of a 2.5 g piece of copper wire from 20°C to 80°C? How much heat is needed to increase the temperature of an equivalent mass of aluminum by the same amount? If you were using one of these metals to channel heat away from electrical components, which metal would you use? Once heated, which metal will cool faster? Give the specific heat for each metal.
-
Gold has a molar heat capacity of 25.418 J/(mol·K), and silver has a molar heat capacity of 23.350 J/(mol·K).
- If you put silver and gold spoons of equal mass into a cup of hot liquid and wait until the temperature of the liquid is constant, which spoon will take longer to cool down when removed from the hot liquid?
- If 8.00 g spoons of each metal at 20.0°C are placed in an insulated mug with 50.0 g of water at 97.0°C, what will be the final temperature of the water after the system has equilibrated? (Assume that no heat is transferred to the surroundings.)
-
In an exothermic reaction, how much heat would need to be evolved to raise the temperature of 150 mL of water 7.5°C? Explain how this process illustrates the law of conservation of energy.
-
How much heat must be evolved by a reaction to raise the temperature of 8.0 oz of water 5.0°C? What mass of lithium iodide would need to be dissolved in this volume of water to produce this temperature change?
-
A solution is made by dissolving 3.35 g of an unknown salt in 150 mL of water, and the temperature of the water rises 3.0°C. The addition of a silver nitrate solution results in a precipitate. Assuming that the heat capacity of the solution is the same as that of pure water, use the information in Table 9.5.1 and solubility rules to identify the salt.
-
Using the data in Table 9.8.2, calculate the change in temperature of a calorimeter with a heat capacity of 1.78 kJ/°C when 3.0 g of charcoal is burned in the calorimeter. If the calorimeter is in a 2 L bath of water at an initial temperature of 21.5°C, what will be the final temperature of the water after the combustion reaction (assuming no heat is lost to the surroundings)?
-
A 3.00 g sample of TNT (trinitrotoluene, C 7 H 5 N 3 O 6 ) is placed in a bomb calorimeter with a heat capacity of 1.93 kJ/°C; the Δ H comb of TNT is −3403.5 kJ/mol. If the initial temperature of the calorimeter is 19.8°C, what will be the final temperature of the calorimeter after the combustion reaction (assuming no heat is lost to the surroundings)? What is the Δ H f of TNT?
Answers
-
C p = C s × (molar mass)
-
-
For Cu: q = 58 J; For Al: q = 130 J; Even though the values of the molar heat capacities are very similar for the two metals, the specific heat of Cu is only about half as large as that of Al, due to the greater molar mass of Cu versus Al: C s = 0.385 and 0.897 J/ (g•K) for Cu and Al, respectively. Thus loss of one joule of heat will cause almost twice as large a decrease in temperature of Cu versus Al.
-
-
4.7 kJ
-
-
Δ H soln = −0.56 kJ/g; based on reaction with AgNO 3 , salt contains halide; dividing Δ H soln values in Table 5.2 by molar mass of salts gives lithium bromide as best match, with −0.56 kJ/g.
-
-
T final = 43.1°C; the combustion reaction is [4C_7H_5N_3O_{6(s)} + 21O_{2(g)} rightarrow 28CO_{2(g)} + 10H_2O_{(g)} + 6N_{2(g)}] with [Δ_f^οH (TNT) = −65.5; kJ/mol]
5.6: Hess’s Law
Conceptual Problems
Please be sure you are familiar with the topics discussed in Essential Skills 4 ( Section 5.6 “Essential Skills 4” ) before proceeding to the Conceptual Problems.
-
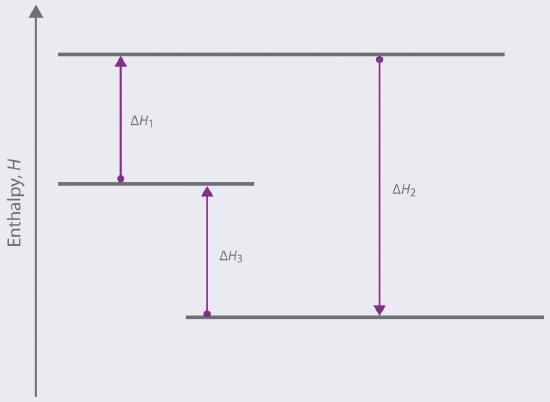
Based on the following energy diagram,
a. write an equation showing how the value of Δ H 2 could be determined if the values of Δ H 1 and Δ H 3 are known.
b; identify each step as being exothermic or endothermic.
-
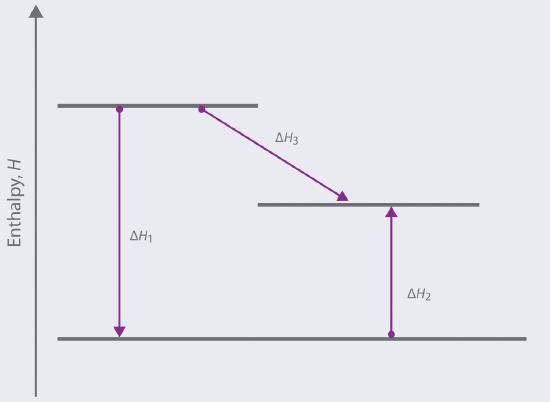
Based on the following energy diagram,
a. write an equation showing how the value of Δ H 3 could be determined if the values of Δ H 1 and Δ H 2 are known.
b. identify each step as being exothermic or endothermic.
-
Describe how Hess’s law can be used to calculate the enthalpy change of a reaction that cannot be observed directly.
-
When you apply Hess’s law, what enthalpy values do you need to account for each change in physical state?
- the melting of a solid
- the conversion of a gas to a liquid
- the solidification of a liquid
- the dissolution of a solid into water
-
In their elemental form, A 2 and B 2 exist as diatomic molecules. Given the following reactions, each with an associated Δ H °, describe how you would calculate ΔH o f for the compound AB 2 .
( begin{matrix}
2AB & rightarrow & A_{2} + B _{2} & Delta H_{1}^{o}\
3AB & rightarrow & AB_{2} + A _{2}B & Delta H_{2}^{o} \
2A_{2}B &rightarrow & 2A_{2} + B _{2} & Delta H_{3}^{o}
end{matrix} )
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 4 before proceeding to the Numerical Problems.
-
Methanol is used as a fuel in Indianapolis 500 race cars. Use the following table to determine whether methanol or 2,2,4-trimethylpentane (isooctane) releases more energy per liter during combustion.
Fuel ΔH o combustion (kJ/mol) Density (g/mL) methanol −726.1 0.791 2,2,4-trimethylpentane −5461.4 0.692 -
a. Use the enthalpies of combustion given in the following table to determine which organic compound releases the greatest amount of energy per gram during combustion.
Fuel ΔH o combustion (kJ/mol)
methanol −726.1 1-ethyl-2-methylbenzene −5210.2 n -octane −5470.5 b. Calculate the standard enthalpy of formation of 1-ethyl-2-methylbenzene.
-
Given the enthalpies of combustion, which organic compound is the best fuel per gram?
Fuel ΔH o f (kJ/mol)
ethanol −1366.8 benzene −3267.6 cyclooctane −5434.7
Numerical Answers
1.
2.
a. To one decimal place
methanol: Δ H /g = −22.6 kJ
C 9 H 12 : Δ H /g = −43.3 kJ
octane: Δ H /g = −47.9 kJ
Octane provides the largest amount of heat per gram upon combustion.
b, Δ H f (C 9 H 17 ) = −46.1 kJ/mol
5.7: Enthalpies of Formation
Conceptual Problems
Please be sure you are familiar with the topics discussed i n Essential Skills 4 ( Section 5.6 “Essential Skills 4” ) befo re proceeding to the Conceptual Problems.
- Describe how Hess’s law can be used to calculate the enthalpy change of a reaction that cannot be observed directly.
- When you apply Hess’s law, what enthalpy values do you need to account for each change in physical state?
- What is the difference between ΔH o f and ΔH f ?
- How can ΔH o f of a compound be determined if the compound cannot be prepared by the reactions used to define its standard enthalpy of formation?
- For the formation of each compound, write a balanced chemical equation corresponding to the standard enthalpy of formation of each compound.
- HBr
- CH 3 OH
- NaHCO 3
- Describe the distinction between Δ H soln and Δ H f .
- The following table lists ΔH o soln values for some ionic compounds. If 1 mol of each solute is dissolved in 500 mL of water, rank the resulting solutions from warmest to coldest.
| Compound | ΔH o soln (kJ/mol) |
|---|---|
| KOH | −57.61 |
| LiNO 3 | −2.51 |
| KMnO 4 | 43.56 |
| NaC 2 H 3 O 2 | −17.32 |
Numerical Problems
Please be sure you are familiar with the topics discussed in Esse ntial Skills 4 ( Section 5.6 “Essential Skills 4” ) before pr oceeding to the Numerical Problems.
-
Using “Appendix A , calculate ΔH o rxn for each chemical reaction.
a. 2Mg(s) + O 2 (g) → 2MgO(s)
b. CaCO 3 (s, calcite) → CaO(s) + CO 2 (g)
c. AgNO 3 (s) + NaCl(s) → AgCl(s) + NaNO 3 (s)
-
Using “Appendix A , determine ΔH o rxn for each chemical reaction.
a. 2Na(s) + Pb(NO 3 ) 2 (s) → 2NaNO 3 (s) + Pb(s)
b. Na 2 CO 3 (s) + H 2 SO 4 (l) → Na 2 SO 4 (s) + CO 2 (g) + H 2 O(l)
c. 2KClO 3 (s) → 2KCl(s) + 3O 2 (g)
-
Calculate ΔH o rxn for each chemical equation. If necessary, balance the chemical equations.
a. Fe(s) + CuCl 2 (s) → FeCl 2 (s) + Cu(s)
b. (NH 4 ) 2 SO 4 (s) + Ca(OH) 2 (s) → CaSO 4 (s) + NH 3 (g) + H 2 O(l)
c. Pb(s) + PbO 2 (s) + H 2 SO 4 (l) → PbSO 4 (s) + H 2 O(l)
-
Calculate ΔH o rxn for each reaction. If necessary, balance the chemical equations.
a. 4HBr(g) + O 2 (g) → 2H 2 O(l) + 2Br 2 (l)
b. 2KBr(s) + H 2 SO 4 (l) → K 2 SO 4 (s) + 2HBr(g)
c. 4Zn(s) + 9HNO 3 (l) → 4Zn(NO 3 ) 2 (s) + NH 3 (g) + 3H 2 O(l)
-
Use the data in “Appendix A to calculate ΔH o f for the reaction Sn(s, white) + 4HNO 3 (l) → SnO 2 (s) + 4NO 2 (g) + 2H 2 O(l).
-
Use the data in “Appendix A to calculate ΔH o f for the reaction P 4 O 10 (s) + 6H 2 O(l) → 4H 3 PO 4 (l).
-
How much heat is released or required in the reaction of 0.50 mol of HBr(g) with 1.0 mol of chlorine gas to produce bromine gas?
-
How much energy is released or consumed if 10.0 g of N 2 O 5 is completely decomposed to produce gaseous nitrogen dioxide and oxygen?
-
In the mid-1700s, a method was devised for preparing chlorine gas from the following reaction:
NaCl(s) + H 2 SO 4 (l) + MnO 2 (s) → Na 2 SO 4 (s) + MnCl 2 (s) + H 2 O(l) + Cl 2 (g)
Calculate ΔH o rxn for this reaction. Is the reaction exothermic or endothermic?
-
Would you expect heat to be evolved during each reaction?
- solid sodium oxide with gaseous sulfur dioxide to give solid sodium sulfite
- solid aluminum chloride reacting with water to give solid aluminum oxide and hydrogen chloride gas
-
How much heat is released in preparing an aqueous solution containing 6.3 g of calcium chloride, an aqueous solution containing 2.9 g of potassium carbonate, and then when the two solutions are mixed together to produce potassium chloride and calcium carbonate.
Numerical Answers
-
a. −1203 kJ/mol O 2
b. 179.2 kJ
c. −59.3 kJ
-
−174.1 kJ/mol
-
−20.3 kJ
-
−34.3 kJ/mol Cl 2 ; exothermic
-
Δ H = −2.86 kJ CaCl 2 : −4.6 kJ; K 2 CO 3 , −0.65 kJ; mixing, −0.28 kJ
5.8: Foods and Fuels
Conceptual Problems
- Can water be considered a food? Explain your answer.
- Describe how you would determine the caloric content of a bag of popcorn using a calorimeter.
- Why do some people initially feel cold after eating a meal and then begin to feel warm?
- In humans, one of the biochemical products of the combustion/digestion of amino acids is urea. What effect does this have on the energy available from these reactions? Speculate why conversion to urea is preferable to the generation of N 2 .
Numerical Problems
Please be sure you are familiar with the topics discusse d in Essential Skills 4 (Section 9.9 ) before proceeding to the Numerical Problems.
-
Determine the amount of energy available from the biological oxidation of 1.50 g of leucine (an amino acid, Δ H comb = −3581.7 kJ/mol).
-
Calculate the energy released (in kilojoules) from the metabolism of 1.5 oz of vodka that is 62% water and 38% ethanol by volume, assuming that the total volume is equal to the sum of the volume of the two components. The density of ethanol is 0.824 g/mL. What is this enthalpy change in nutritional Calories?
-
While exercising, a person lifts an 80 lb barbell 7 ft off the ground. Assuming that the transformation of chemical energy to mechanical energy is only 35% efficient, how many Calories would the person use to accomplish this task? From Figure 9.4.2, how many grams of glucose would be needed to provide the energy to accomplish this task?
-
A 30 g sample of potato chips is placed in a bomb calorimeter with a heat capacity of 1.80 kJ/°C, and the bomb calorimeter is immersed in 1.5 L of water. Calculate the energy contained in the food per gram if, after combustion of the chips, the temperature of the calorimeter increases to 58.6°C from an initial temperature of 22.1°C.