Estos son ejercicios de tarea para acompañar el Mapa de texto creado para “Química: La ciencia central” por Brown et al. Se pueden encontrar bancos de preguntas de química general complementaria para otros mapas de texto y se puede acceder aquí . Además de estas preguntas disponibles públicamente, el acceso al banco privado de problemas para su uso en exámenes y tareas está disponible para los profesores solo de manera individual; comuníquese con Delmar Larsen para obtener una cuenta con permiso de acceso.
4.1: Propiedades generales de las soluciones acuosas
Problemas conceptuales
-
¿Cuáles son las ventajas de llevar a cabo una reacción en solución en lugar de simplemente mezclar los reactivos puros?
-
¿Qué tipos de compuestos se disuelven en solventes polares?
-
Describa la distribución de carga en agua líquida. ¿Cómo afecta esta distribución a sus propiedades físicas?
-
¿Debe una molécula tener una distribución de carga asimétrica para ser polar? Explica tu respuesta.
-
¿Por qué muchas sustancias iónicas son solubles en agua?
-
Explica la frase like disuelve like .
-
¿Qué tipos de compuestos covalentes son solubles en agua?
-
¿Por qué la mayoría de los hidrocarburos aromáticos tienen una solubilidad limitada en agua? ¿Esperarías que su solubilidad sea mayor, menor o igual en etanol en comparación con el agua? ¿Por qué?
-
Predice si cada compuesto se disolverá en agua y explica por qué.
- tolueno
- ácido acético
- acetato de sodio
- butanol
- ácido pentanoico
-
Predice si cada compuesto se disolverá en agua y explica por qué.
- cloruro de amonio
- 2-propanol
- heptano
- dicromato de potasio
- 2-octanol
-
Dado el agua y el tolueno, predice cuál es el mejor solvente para cada compuesto y explica tu razonamiento.
- cianuro de sodio
- benceno
- ácido acético
- Etóxido de sodio (CH 3 CH 2 ONa)
-
De agua y tolueno, predice cuál es el mejor solvente para cada compuesto y explica tu razonamiento.
- t -butanol
- cloruro de calcio
- sacarosa
- ciclohexeno
-
Compuesto A se divide en tres muestras iguales. La primera muestra no se disuelve en agua, la segunda muestra se disuelve solo ligeramente en etanol y la tercera muestra se disuelve completamente en tolueno. ¿Qué sugiere esto sobre la polaridad de A ?
-
Se le da una mezcla de tres compuestos sólidos: A , B y C y se le dice que A es un compuesto polar, B es ligeramente polar, y C es no polar. Sugiera un método para separar estos tres compuestos.
-
Un técnico de laboratorio recibe una muestra que contiene solo cloruro de sodio, sacarosa y ciclodecanona (una cetona). Debe decirle al técnico cómo separar estos tres compuestos de la mezcla. ¿Qué sugieres?
-
Muchos medicamentos de venta libre se venden como soluciones de etanol / agua en lugar de soluciones puramente acuosas. Dé una razón plausible para esta práctica.
-
¿Qué distingue un electrolito débil de un electrolito fuerte?
-
¿Qué grupos orgánicos dan como resultado soluciones acuosas que conducen electricidad?
-
Se considera altamente peligroso salpicar descalzo en los charcos durante una tormenta eléctrica. ¿Por qué?
-
¿Qué solución (es) esperaría para conducir bien la electricidad? Explica tu razonamiento.
- una solución acuosa de cloruro de sodio
- una solución de etanol en agua
- una solución de cloruro de calcio en agua
- una solución de sacarosa en agua
-
¿Qué solución (es) esperaría para conducir bien la electricidad? Explica tu razonamiento.
- una solución acuosa de ácido acético
- una solución acuosa de hidróxido de potasio
- una solución de etilenglicol en agua
- una solución de cloruro de amonio en agua
-
¿Cuál de los siguientes es un electrolito fuerte, un electrolito débil o un no electrolito en una solución acuosa? Explica tu razonamiento.
- hidróxido de potasio
- amoníaco
- cloruro de calcio
- ácido butanoico
-
¿Cuál de los siguientes es un electrolito fuerte, un electrolito débil o un no electrolito en una solución acuosa? Explica tu razonamiento.
- hidróxido de magnesio
- butanol
- bromuro de amonio
- ácido pentanoico
-
¿Cuál de los siguientes es un electrolito fuerte, un electrolito débil o un no electrolito en solución acuosa? Explica tu razonamiento.
- H 2 SO 4
- dietilamina
- 2-propanol
- cloruro de amonio
- ácido propanoico
Respuestas conceptuales
-
Ionic compounds such as NaCl are held together by electrostatic interactions between oppositely charged ions in the highly ordered solid. When an ionic compound dissolves in water, the partially negatively charged oxygen atoms of the H 2 O molecules surround the cations, and the partially positively charged hydrogen atoms in H 2 O surround the anions. The favorable electrostatic interactions between water and the ions compensate for the loss of the electrostatic interactions between ions in the solid.
-
- Because toluene is an aromatic hydrocarbon that lacks polar groups, it is unlikely to form a homogenous solution in water.
- Acetic acid contains a carboxylic acid group attached to a small alkyl group (a methyl group). Consequently, the polar characteristics of the carboxylic acid group will be dominant, and acetic acid will form a homogenous solution with water.
- Because most sodium salts are soluble, sodium acetate should form a homogenous solution with water.
- Like all alcohols, butanol contains an −OH group that can interact well with water. The alkyl group is rather large, consisting of a 4-carbon chain. In this case, the nonpolar character of the alkyl group is likely to be as important as the polar character of the –OH, decreasing the likelihood that butanol will form a homogeneous solution with water.
- Like acetic acid, pentanoic acid is a carboxylic acid. Unlike acetic acid, however, the alkyl group is rather large, consisting of a 4-carbon chain as in butanol. As with butanol, the nonpolar character of the alkyl group is likely to be as important as the polar character of the carboxylic acid group, making it unlikely that pentanoic acid will form a homogeneous solution with water. (In fact, the solubility of both butanol and pentanoic acid in water is quite low, only about 3 g per 100 g water at 25°C.)
-
An electrolyte is any compound that can form ions when it dissolves in water. When a strong electrolyte dissolves in water, it dissociates completely to give the constituent ions. In contrast, when a weak electrolyte dissolves in water, it produces relatively few ions in solution.
4.2: Precipitation Reactions
Conceptual Problems
-
What information can be obtained from a complete ionic equation that cannot be obtained from the overall chemical equation?
-
Predict whether mixing each pair of solutions will result in the formation of a precipitate. If so, identify the precipitate.
- FeCl 2 (aq) + Na 2 S(aq)
- NaOH(aq) + H 3 PO 4 (aq)
- ZnCl 2 (aq) + (NH 4 ) 2 S(aq)
-
Predict whether mixing each pair of solutions will result in the formation of a precipitate. If so, identify the precipitate.
- KOH(aq) + H 3 PO 4 (aq)
- K 2 CO 3 (aq) + BaCl 2 (aq)
- Ba(NO 3 ) 2 (aq) + Na 2 SO 4 (aq)
-
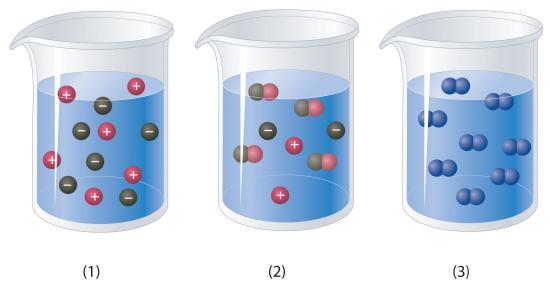
Which representation best corresponds to an aqueous solution originally containing each of the following?
- 1 M NH 4 Cl
- 1 M NaO 2 CCH 3
- 1 M NaOH + 1 M HCl
-
1 M Ba(OH) 2 + 1 M H 2 SO 4
-
Which representation in Problem 3 best corresponds to an aqueous solution originally containing each of the following?
- 1 M CH 3 CO 2 H + 1 M NaOH
- 1 M NH 3 + 1 M HCl
- 1 M Na 2 CO 3 + 1 M H 2 SO 4
- 1 M CaCl 2 + 1 M H 3 PO 4
Conceptual Answer
3
- 1
- 1
- 1
- 2
Numerical Problems
-
What mass of precipitate would you expect to obtain by mixing 250 mL of a solution containing 4.88 g of Na 2 CrO 4 with 200 mL of a solution containing 3.84 g of AgNO 3 ? What is the final nitrate ion concentration?
-
Adding 10.0 mL of a dilute solution of zinc nitrate to 246 mL of 2.00 M sodium sulfide produced 0.279 g of a precipitate. How many grams of zinc(II) nitrate and sodium sulfide were consumed to produce this quantity of product? What was the concentration of each ion in the original solutions? What is the concentration of the sulfide ion in solution after the precipitation reaction, assuming no further reaction?
Numerical Answer
-
3.75 g Ag 2 CrO 4 ; 5.02 × 10 −2 M nitrate
4.3: Acid-Base Reactions
Conceptual Problems
-
Why was it necessary to expand on the Arrhenius definition of an acid and a base? What specific point does the Brønsted–Lowry definition address?
-
State whether each compound is an acid, a base, or a salt.
- CaCO 3
- NaHCO 3
- H 2 SO 4
- CaCl 2
- Ba(OH) 2
-
State whether each compound is an acid, a base, or a salt.
- NH 3
- NH 4 Cl
- H 2 CO 3
- CH 3 COOH
- NaOH
-
Classify each compound as a strong acid, a weak acid, a strong base, or a weak base in aqueous solution.
- sodium hydroxide
- acetic acid
- magnesium hydroxide
- tartaric acid
- sulfuric acid
- ammonia
- hydroxylamine (NH 2 OH)
- hydrocyanic acid
-
Decide whether each compound forms an aqueous solution that is strongly acidic, weakly acidic, strongly basic, or weakly basic.
- propanoic acid
- hydrobromic acid
- methylamine
- lithium hydroxide
- citric acid
- sodium acetate
- ammonium chloride
- barium hydroxide
-
What is the relationship between the strength of an acid and the strength of the conjugate base derived from that acid? Would you expect the CH 3 CO 2 − ion to be a strong base or a weak base? ¿Por qué? Is the hydronium ion a strong acid or a weak acid? Explain your answer.
-
What are the products of an acid–base reaction? Under what circumstances is one of the products a gas?
-
Explain how an aqueous solution that is strongly basic can have a pH, which is a measure of the acidity of a solution.
Answer
-
- weakly acidic
- strongly acidic
- weakly basic
- strongly basic
- weakly acidic
- weakly basic
- weakly acidic
- strongly basic
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 3 ( section 4.1 “Aqueous Solutions” 0) before proceeding to the Numerical Problems.
-
Derive an equation to relate the hydrogen ion concentration to the molarity of a solution of a strong monoprotic acid.
-
Derive an equation to relate the hydroxide ion concentration to the molarity of a solution of
- a group I hydroxide.
- a group II hydroxide.
-
Given the following salts, identify the acid and the base in the neutralization reactions and then write the complete ionic equation:
- barium sulfate
- lithium nitrate
- sodium bromide
- calcium perchlorate
-
What is the pH of each solution?
- 5.8 × 10 −3 mol of HNO 3 in 257 mL of water
- 0.0079 mol of HI in 750 mL of water
- 0.011 mol of HClO 4 in 500 mL of water
- 0.257 mol of HBr in 5.00 L of water
-
What is the hydrogen ion concentration of each substance in the indicated pH range?
- black coffee (pH 5.10)
- milk (pH 6.30–7.60)
- tomatoes (pH 4.00–4.40)
-
What is the hydrogen ion concentration of each substance in the indicated pH range?
- orange juice (pH 3–4)
- fresh egg white (pH 7.60–7.80)
- lemon juice (pH 2.20–2.40)
-
What is the pH of a solution prepared by diluting 25.00 mL of 0.879 M HCl to a volume of 555 mL?
-
Vinegar is primarily an aqueous solution of acetic acid. Commercial vinegar typically contains 5.0 g of acetic acid in 95.0 g of water. What is the concentration of commercial vinegar? If only 3.1% of the acetic acid dissociates to CH 3 CO 2 − and H + , what is the pH of the solution? (Assume the density of the solution is 1.00 g/mL.)
-
If a typical household cleanser is 0.50 M in strong base, what volume of 0.998 M strong monoprotic acid is needed to neutralize 50.0 mL of the cleanser?
-
A 25.00 mL sample of a 0.9005 M solution of HCl is diluted to 500.0 mL. What is the molarity of the final solution? How many milliliters of 0.223 M NaOH are needed to neutralize 25.00 mL of this final solution?
-
If 20.0 mL of 0.10 M NaOH are needed to neutralize 15.0 mL of gastric fluid, what is the molarity of HCl in the fluid? (Assume all the acidity is due to the presence of HCl.) What other base might be used instead of NaOH?
-
Malonic acid (C 3 H 4 O 4 ) is a diprotic acid used in the manufacture of barbiturates. How many grams of malonic acid are in a 25.00 mL sample that requires 32.68 mL of 1.124 M KOH for complete neutralization to occur? Malonic acid is a dicarboxylic acid; propose a structure for malonic acid.
-
Describe how you would prepare 500 mL of a 1.00 M stock solution of HCl from an HCl solution that is 12.11 M. Using your stock solution, how would you prepare 500 mL of a solution that is 0.012 M in HCl?
-
Given a stock solution that is 8.52 M in HBr, describe how you would prepare a 500 mL solution with each concentration.
- 2.50 M
- 4.00 × 10 −3 M
- 0.989 M
-
How many moles of solute are contained in each?
- 25.00 mL of 1.86 M NaOH
- 50.00 mL of 0.0898 M HCl
- 13.89 mL of 0.102 M HBr
-
A chemist needed a solution that was approximately 0.5 M in HCl but could measure only 10.00 mL samples into a 50.00 mL volumetric flask. Propose a method for preparing the solution. (Assume that concentrated HCl is 12.0 M.)
-
Write the balanced chemical equation for each reaction.
- perchloric acid with potassium hydroxide
- nitric acid with calcium hydroxide
-
Write the balanced chemical equation for each reaction.
- solid strontium hydroxide with hydrobromic acid
- aqueous sulfuric acid with solid sodium hydroxide
-
A neutralization reaction gives calcium nitrate as one of the two products. Identify the acid and the base in this reaction. What is the second product? If the product had been cesium iodide, what would have been the acid and the base? What is the complete ionic equation for each reaction?
Answers
-
[H 3 O + ] = [HA] M
-
- H 2 SO 4 and Ba(OH) 2 ; 2H + + SO 4 2− + Ba 2 + + 2OH − → 2H 2 O + Ba 2 + + SO 4 2−
- HNO 3 and LiOH; H + + NO 3 − + Li + + OH − → H 2 O + Li + + NO 3 −
- HBr and NaOH; H + + Br − + Na + + OH − → H 2 O + Na + + Br −
- HClO 4 and Ca(OH) 2 ; 2H + + 2ClO 4 − + Ca 2 + + 2OH − → 2H 2 O + Ca 2 + + 2ClO 4 −
-
- 7.9 × 10 −6 M H +
- 5.0 × 10 −7 to 2.5 × 10 −8 M H +
- 1.0 × 10 −4 to 4.0 × 10 −5 M H +
-
pH = 1.402
-
25 mL
-
0.13 M HCl; magnesium carbonate, MgCO 3 , or aluminum hydroxide, Al(OH) 3
-
1.00 M solution: dilute 41.20 mL of the concentrated solution to a final volume of 500 mL. 0.012 M solution: dilute 12.0 mL of the 1.00 M stock solution to a final volume of 500 mL.
-
- 4.65 × 10 −2 mol NaOH
- 4.49 × 10 −3 mol HCl
- 1.42 × 10 −3 mol HBr
-
- HClO 4 + KOH → KClO 4 + H 2 O
- 2HNO 3 + Ca(OH) 2 → Ca(NO 3 ) 2 + 2H 2 O
-
The acid is nitric acid, and the base is calcium hydroxide. The other product is water.
(2HNO_3 + Ca(OH)_2 rightarrow Ca(NO_3)_2 + 2H_2O)
The acid is hydroiodic acid, and the base is cesium hydroxide. The other product is water.
( HI + CsOH rightarrow CsI + H_2O )
The complete ionic equations are
( 2H^+ + 2NO_3^- + Ca^{2+} + 2OH^- rightarrow Ca^{2+} + 2NO_3^- + H_2O)
( H^+ + I^- + Cs^+ + OH^- rightarrow Cs^+ + I^- + H_2O )
4.4: Oxidation-Reduction Reactions
Conceptual Problems
-
Which elements in the periodic table tend to be good oxidants? Which tend to be good reductants?
-
If two compounds are mixed, one containing an element that is a poor oxidant and one with an element that is a poor reductant, do you expect a redox reaction to occur? Explain your answer. What do you predict if one is a strong oxidant and the other is a weak reductant? ¿Por qué?
-
In each redox reaction, determine which species is oxidized and which is reduced:
- Zn(s) + H 2 SO 4 (aq) → ZnSO 4 (aq) + H 2 (g)
- Cu(s) + 4HNO 3 (aq) → Cu(NO 3 ) 2 (aq) + 2NO 2 (g) + 2H 2 O(l)
- BrO 3 − (aq) + 2MnO 2 (s) + H 2 O(l) → Br − (aq) + 2MnO 4 − (aq) + 2H + (aq)
-
Single-displacement reactions are a subset of redox reactions. In this subset, what is oxidized and what is reduced? Give an example of a redox reaction that is not a single-displacement reaction.
Numerical Problems
-
Balance each redox reaction under the conditions indicated.
- CuS(s) + NO 3 − (aq) → Cu 2 + (aq) + SO 4 2− (aq) + NO(g); acidic solution
- Ag(s) + HS − (aq) + CrO 4 2− (aq) → Ag 2 S(s) + Cr(OH) 3 (s); basic solution
- Zn(s) + H 2 O(l) → Zn 2 + (aq) + H 2 (g); acidic solution
- O 2 (g) + Sb(s) → H 2 O 2 (aq) + SbO 2 − (aq); basic solution
- UO 2 2 + (aq) + Te(s) → U 4 + (aq) + TeO 4 2− (aq); acidic solution
-
Balance each redox reaction under the conditions indicated.
- MnO 4 − (aq) + S 2 O 3 2− (aq) → Mn 2 + (aq) + SO 4 2− (aq); acidic solution
- Fe 2 + (aq) + Cr 2 O 7 2− (aq) → Fe 3 + (aq) + Cr 3 + (aq); acidic solution
- Fe(s) + CrO 4 2− (aq) → Fe 2 O 3 (s) + Cr 2 O 3 (s); basic solution
- Cl 2 (aq) → ClO 3 − (aq) + Cl − (aq); acidic solution
- CO 3 2− (aq) + N 2 H 4 (aq) → CO(g) + N 2 (g); basic solution
-
Using the activity series, predict what happens in each situation. If a reaction occurs, write the net ionic equation; then write the complete ionic equation for the reaction.
- Platinum wire is dipped in hydrochloric acid.
- Manganese metal is added to a solution of iron(II) chloride.
- Tin is heated with steam.
- Hydrogen gas is bubbled through a solution of lead(II) nitrate.
-
Using the activity series, predict what happens in each situation. If a reaction occurs, write the net ionic equation; then write the complete ionic equation for the reaction.
- A few drops of NiBr 2 are dropped onto a piece of iron.
- A strip of zinc is placed into a solution of HCl.
- Copper is dipped into a solution of ZnCl 2 .
- A solution of silver nitrate is dropped onto an aluminum plate.
-
Dentists occasionally use metallic mixtures called amalgams for fillings. If an amalgam contains zinc, however, water can contaminate the amalgam as it is being manipulated, producing hydrogen gas under basic conditions. As the filling hardens, the gas can be released, causing pain and cracking the tooth. Write a balanced chemical equation for this reaction.
-
Copper metal readily dissolves in dilute aqueous nitric acid to form blue Cu 2 + (aq) and nitric oxide gas.
- What has been oxidized? What has been reduced?
- Balance the chemical equation.
-
Classify each reaction as an acid–base reaction, a precipitation reaction, or a redox reaction, or state if there is no reaction; then complete and balance the chemical equation:
- Pt 2 + (aq) + Ag(s) →
- HCN(aq) + NaOH(aq) →
- Fe(NO 3 ) 3 (aq) + NaOH(aq) →
- CH 4 (g) + O 2 (g) →
-
Classify each reaction as an acid–base reaction, a precipitation reaction, or a redox reaction, or state if there is no reaction; then complete and balance the chemical equation:
- Zn(s) + HCl(aq) →
- HNO 3 (aq) + AlCl 3 (aq) →
- K 2 CrO 4 (aq) + Ba(NO 3 ) 2 (aq) →
- Zn(s) + Ni 2 + (aq) → Zn 2 + (aq) + Ni(s)
4.5: Concentration of Solutions
Conceptual Problems
-
Which of the representations best corresponds to a 1 M aqueous solution of each compound? Justify your answers.
- NH 3
- HF
- CH 3 CH 2 CH 2 OH
-
Na 2 SO 4
-
Which of the representations shown in Problem 1 best corresponds to a 1 M aqueous solution of each compound? Justify your answers.
- CH 3 CO 2 H
- NaCl
- Na 2 S
- Na 3 PO 4
- acetaldehyde
-
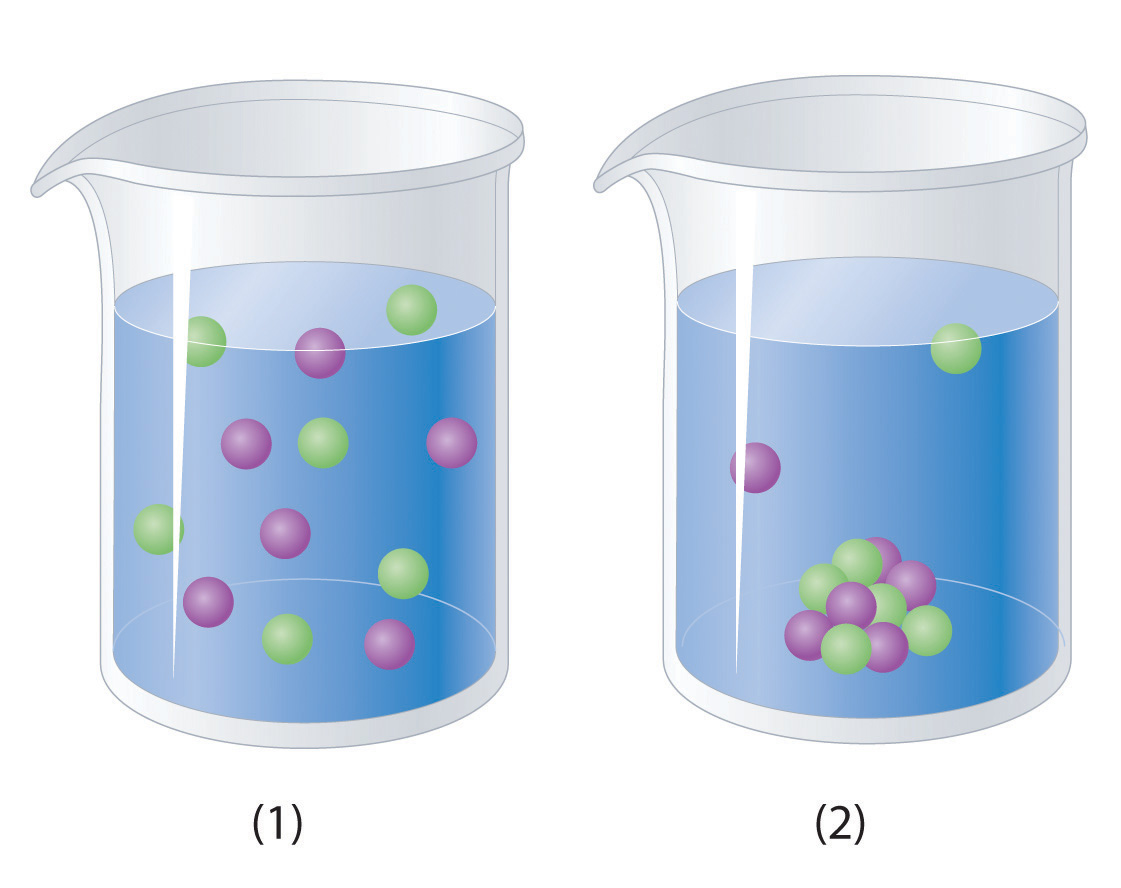
Would you expect a 1.0 M solution of CaCl 2 to be a better conductor of electricity than a 1.0 M solution of NaCl? Why or why not?
-
An alternative way to define the concentration of a solution is molality , abbreviated m . Molality is defined as the number of moles of solute in 1 kg of solvent . How is this different from molarity? Would you expect a 1 M solution of sucrose to be more or less concentrated than a 1 m solution of sucrose? Explain your answer.
-
What are the advantages of using solutions for quantitative calculations?
Conceptual Answer
-
-
-
-
-
If the amount of a substance required for a reaction is too small to be weighed accurately, the use of a solution of the substance, in which the solute is dispersed in a much larger mass of solvent, allows chemists to measure the quantity of the substance more accurately.
Numerical Problems
-
Calculate the number of grams of solute in 1.000 L of each solution.
- 0.2593 M NaBrO 3
- 1.592 M KNO 3
- 1.559 M acetic acid
- 0.943 M potassium iodate
-
Calculate the number of grams of solute in 1.000 L of each solution.
- 0.1065 M BaI 2
- 1.135 M Na 2 SO 4
- 1.428 M NH 4 Br
- 0.889 M sodium acetate
-
If all solutions contain the same solute, which solution contains the greater mass of solute?
- 1.40 L of a 0.334 M solution or 1.10 L of a 0.420 M solution
- 25.0 mL of a 0.134 M solution or 10.0 mL of a 0.295 M solution
- 250 mL of a 0.489 M solution or 150 mL of a 0.769 M solution
-
Complete the following table for 500 mL of solution.
Compound Mass (g) Moles Concentration (M) calcium sulfate 4.86 acetic acid 3.62 hydrogen iodide dihydrate 1.273 barium bromide 3.92 glucose 0.983 sodium acetate 2.42 -
What is the concentration of each species present in the following aqueous solutions?
- 0.489 mol of NiSO 4 in 600 mL of solution
- 1.045 mol of magnesium bromide in 500 mL of solution
- 0.146 mol of glucose in 800 mL of solution
- 0.479 mol of CeCl 3 in 700 mL of solution
-
What is the concentration of each species present in the following aqueous solutions?
- 0.324 mol of K 2 MoO 4 in 250 mL of solution
- 0.528 mol of potassium formate in 300 mL of solution
- 0.477 mol of KClO 3 in 900 mL of solution
- 0.378 mol of potassium iodide in 750 mL of solution
-
What is the molar concentration of each solution?
- 8.7 g of calcium bromide in 250 mL of solution
- 9.8 g of lithium sulfate in 300 mL of solution
- 12.4 g of sucrose (C 12 H 22 O 11 ) in 750 mL of solution
- 14.2 g of iron(III) nitrate hexahydrate in 300 mL of solution
-
What is the molar concentration of each solution?
- 12.8 g of sodium hydrogen sulfate in 400 mL of solution
- 7.5 g of potassium hydrogen phosphate in 250 mL of solution
- 11.4 g of barium chloride in 350 mL of solution
- 4.3 g of tartaric acid (C 4 H 6 O 6 ) in 250 mL of solution
-
Give the concentration of each reactant in the following equations, assuming 20.0 g of each and a solution volume of 250 mL for each reactant.
- BaCl 2 (aq) + Na 2 SO 4 (aq) →
- Ca(OH) 2 (aq) + H 3 PO 4 (aq) →
- Al(NO 3 ) 3 (aq) + H 2 SO 4 (aq) →
- Pb(NO 3 ) 2 (aq) + CuSO 4 (aq) →
- Al(CH 3 CO 2 ) 3 (aq) + NaOH(aq) →
-
An experiment required 200.0 mL of a 0.330 M solution of Na 2 CrO 4 . A stock solution of Na 2 CrO 4 containing 20.0% solute by mass with a density of 1.19 g/cm 3 was used to prepare this solution. Describe how to prepare 200.0 mL of a 0.330 M solution of Na 2 CrO 4 using the stock solution.
-
Calcium hypochlorite [Ca(OCl) 2 ] is an effective disinfectant for clothing and bedding. If a solution has a Ca(OCl) 2 concentration of 3.4 g per 100 mL of solution, what is the molarity of hypochlorite?
-
Phenol (C 6 H 5 OH) is often used as an antiseptic in mouthwashes and throat lozenges. If a mouthwash has a phenol concentration of 1.5 g per 100 mL of solution, what is the molarity of phenol?
-
If a tablet containing 100 mg of caffeine (C 8 H 10 N 4 O 2 ) is dissolved in water to give 10.0 oz of solution, what is the molar concentration of caffeine in the solution?
-
A certain drug label carries instructions to add 10.0 mL of sterile water, stating that each milliliter of the resulting solution will contain 0.500 g of medication. If a patient has a prescribed dose of 900.0 mg, how many milliliters of the solution should be administered?
Numerical Answers
-
-
-
-
-
-
-
-
-
-
-
0.48 M ClO −
-
-
1.74 × 10 −3 M caffeine
4.6: Solution Stoichiometry and Chemical Analysis
Conceptual Problems
-
The titration procedure is an application of the use of limiting reactants. Explain why this is so.
-
Explain how to determine the concentration of a substance using a titration.
-
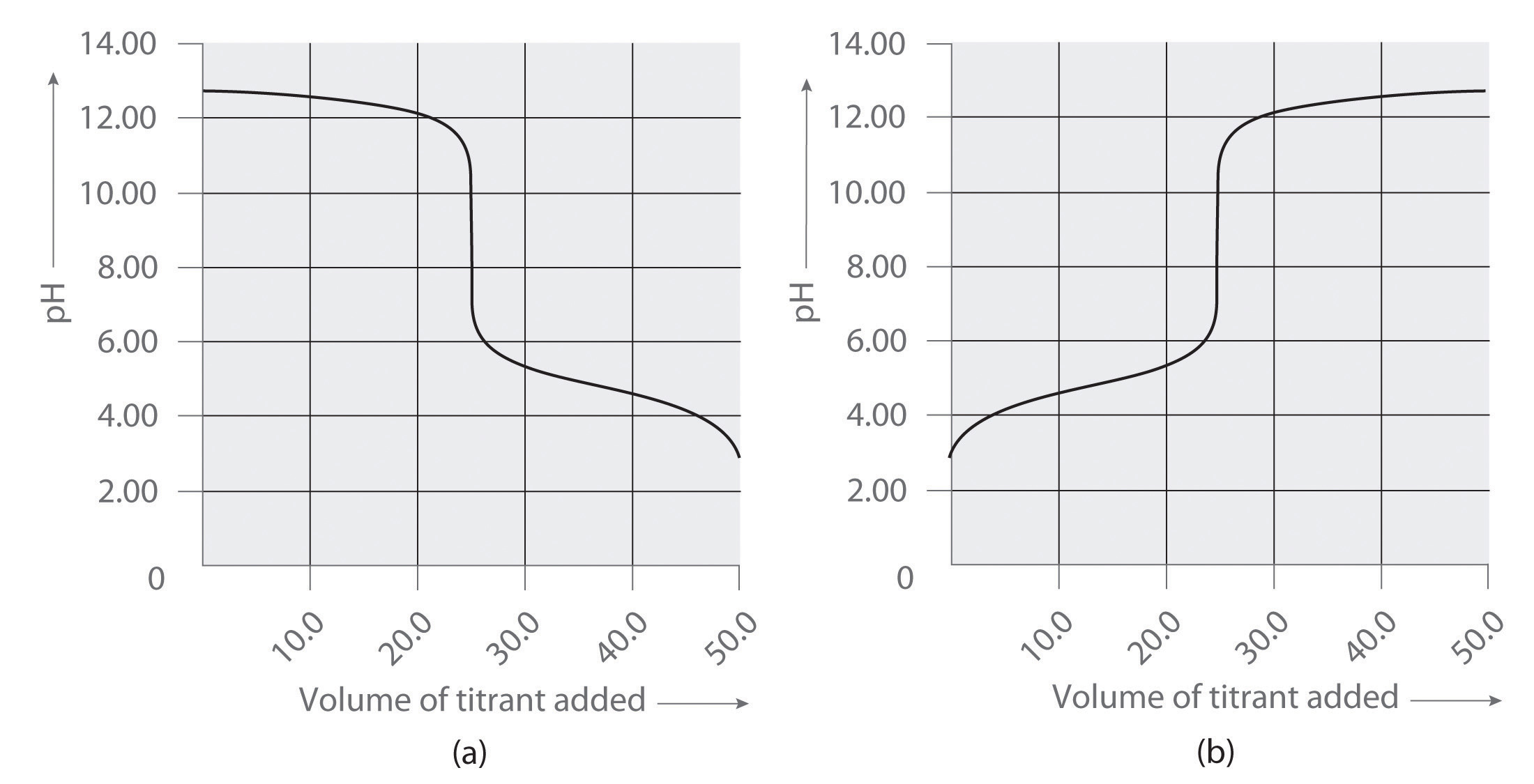
Following are two graphs that illustrate how the pH of a solution varies during a titration. One graph corresponds to the titration of 100 mL 0.10 M acetic acid with 0.10 M NaOH, and the other corresponds to the titration of 100 mL 0.10 M NaOH with 0.10 M acetic acid. Which graph corresponds to which titration? Justify your answer.
-
Following are two graphs that illustrate how the pH of a solution varies during a titration. One graph corresponds to the titration of 100 mL 0.10 M ammonia with 0.10 M HCl, and the other corresponds to the titration of 100 mL 0.10 M NH 4 Cl with 0.10 M NaOH. Which graph corresponds to which titration? Justify your answer.
-
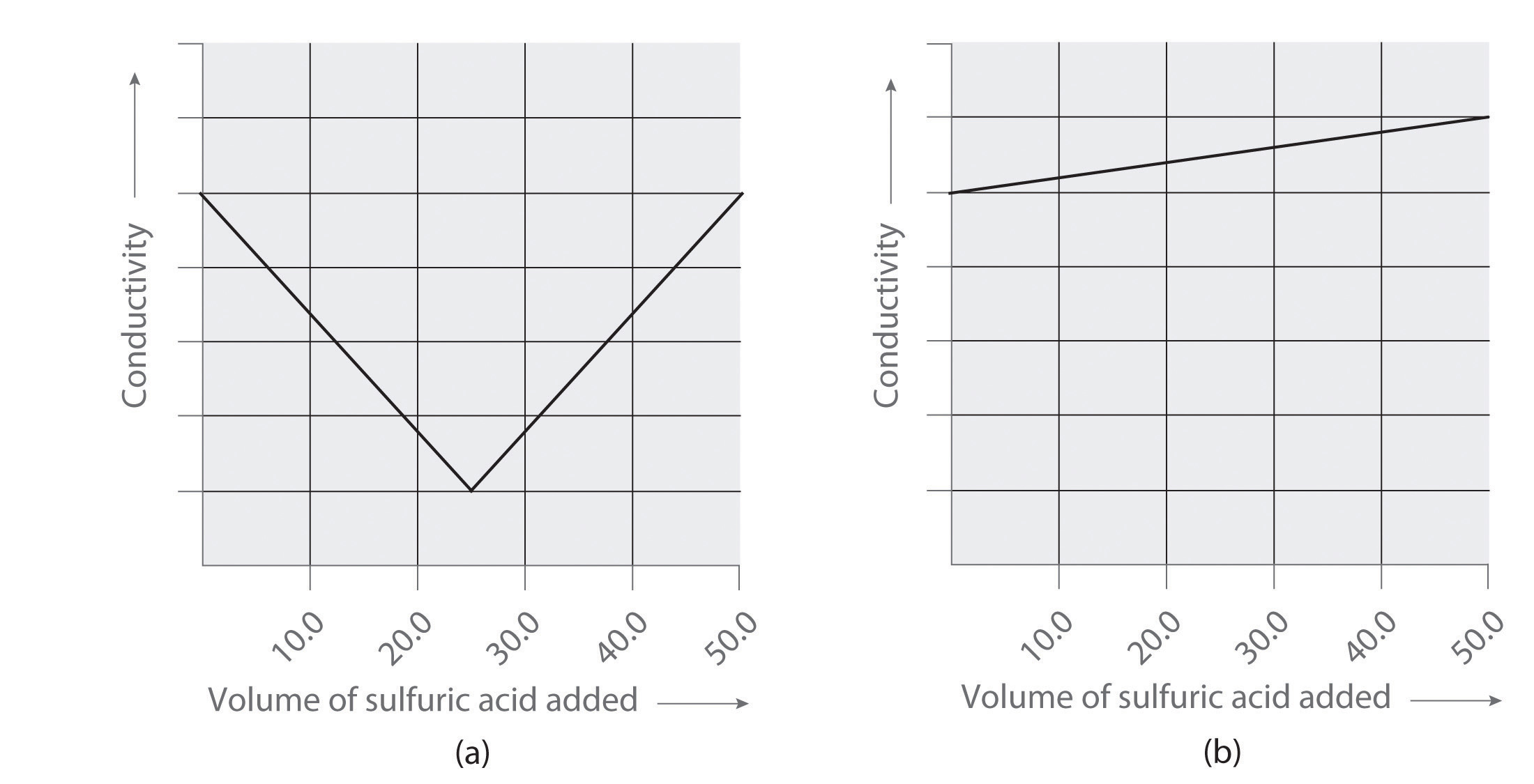
Following are two graphs that illustrate how the electrical conductivity of a solution varies during a titration. One graph corresponds to the titration of 100 mL 0.10 M Ba(OH) 2 with 0.10 M H 2 SO 4 , and the other corresponds to the titration of 100 mL of 0.10 M NaOH with 0.10 M H 2 SO 4 . Which graph corresponds to which titration? Justify your answer.
Conceptual Answers
-
- titration of NaOH with acetic acid
- titration of acetic acid with NaOH
-
- titration of Ba(OH) 2 with sulfuric acid
- titration of NaOH with sulfuric acid
Numerical Problems
-
A 10.00 mL sample of a 1.07 M solution of potassium hydrogen phthalate (KHP, formula mass = 204.22 g/mol) is diluted to 250.0 mL. What is the molarity of the final solution? How many grams of KHP are in the 10.00 mL sample?
-
What volume of a 0.978 M solution of NaOH must be added to 25.0 mL of 0.583 M HCl to completely neutralize the acid? How many moles of NaOH are needed for the neutralization?
-
A student was titrating 25.00 mL of a basic solution with an HCl solution that was 0.281 M. The student ran out of the HCl solution after having added 32.46 mL, so she borrowed an HCl solution that was labeled as 0.317 M. An additional 11.5 mL of the second solution was needed to complete the titration. What was the concentration of the basic solution?